
�Ի�����Ϊԭ��������������������к�Fe2O3��SiO2��Al2O3��MgO�ȡ�ʵ����ģ�ҵ���������Ʊ����죨Fe2O3�����������£�
��1���������ijɷ�������������������� ��д�����ܹ���Fe2O3��ϡ���ᷴӦ�����ӷ�Ӧ����ʽ ��
��2�����������У�Ϊ��ȷ������Ĵ��ȣ�����������Ҫ������Һ��pH�ķ�Χ�� ������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 |
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�ij���������Al��(NH4)2SO4��MgCl2��AlCl3��FeCl2�е�һ�ֻ�����ɣ���ȡ�ù����������ʵ�飬����������й�����������ʾ(������������ݾ�������ɱ�״����)��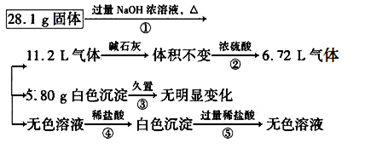
��ش��������⣺
��1����������Ƿ����FeCl2��________(����ڡ������ڡ�)��
��2����������Ƿ����(NH4)2SO4��______(����ڡ������ڡ�)������ж�������____________��
��3��д����Ӧ�ܵ����ӷ���ʽ��____________________��
��4������ݼ����жϻ�������Ƿ����AlCl3��_____(д������ж����ݣ�������д�������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʽ������[Fe(OH)SO4]��һ��������ˮ����������Ч����������ҵ�����÷���м�����������������������ȣ�������ʽ�������Ĺ����������£�
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E�dz�����������ʣ�������ת����ϵ(��ȥ��������Ʒ)��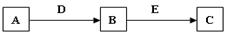
��1����DΪ������CΪNaAlO2����д��Bת��ΪC�Ļ�ѧ����ʽ ��
��2����AΪ��̬���ʣ�BΪƯ�۵���Ч�ɷݣ�C����Ư���ԡ���дAת��ΪB�Ļ�ѧ����ʽ ��������ɵ���A��Ԫ��ԭ�ӽṹʾ��ͼ ��
��3����A��B��C��Ϊ���ε�ˮ��Һ��D��E��Ϊ±�ص��ʣ���д��Bת��ΪC�����ӷ���ʽ ��
��4����A������Ϊ��̬���ʣ�������B�ڳ��³�ѹ��Ϊ���壬B��C����Է�������֮��Ϊ4:5����D��EΪͬһ���ʣ���BΪ ��
��5����B��DΪ������С��18��Ԫ��ԭ���γɵĵ��ʣ�A��E��C��Ϊ�������D����Ԫ��ԭ�ӵ���������B��2����Aת��ΪB��Bת��ΪC������Ӧ�����û���Ӧ����д��Aת��ΪB�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��W��Ԫ�����ڱ���ԭ������������������ֶ����ڣ��������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X������������Ӧ��ˮ���ﻯѧʽΪH2XO3 |
| Y | Y�ǵؿ��к�����ߵ�Ԫ�� |
| Z | Z�Ļ�̬ԭ�����������Ų�ʽΪ3s23p1 |
| W | W��һ�ֺ��ص�������Ϊ28��������Ϊ14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ѧʽΪNa2CO3����һ����Ҫ�Ļ���ԭ�ϡ��ִ��������������ֹ��գ�
һ�������Ƽ����ʳ��Ϊԭ���Ƽ�÷���������
�����Ȼ��������ᷴӦ�������ƣ�2NaCl+H2SO4=Na2SO4+2HCl��
���ý�̿��ԭ�����Ƶ����ƣ�Na2SO4+4C=Na2S+4CO��
����������ʯ��ʯ��Ӧ��̼���ƣ�Na2S+CaCO3=Na2CO3+CaS
�������������ά�Ƽ����ʳ�Ρ�����������̼Ϊԭ�ϣ��䷴ӦҲ���������У�
��NH3+CO2+H2O=NH4HCO3
��NH4HCO3+NaCl=NaHCO3+NH4Cl
��2NaHCO3=Na2CO3+CO2��+H2O
���������Ƽ������������ʳ��ˮ��ͨ�백������ͨ�������̼������̼�����ƣ��ټ���ϸ��ĩ����ͬ����ЧӦ�������Ȼ���ܽ��ͻȻ���ͣ���ʳ�ε��ܽ�ȱ仯���������Ȼ��������ʳ�β����������ð����ͺ�ͨ������̼�������������NaHCO3��NH4Cl���÷������Ĵ�������������������ѩ��
��1��ͨ�����ַ����ıȽϣ������Ƽ���յ�ȱ���� (д����)��
��2��������յ���ѭ�����õ������� (�ѧʽ)����Ʒ�ĸ�����NH4Cl�ȿ����������ֿ����������ɰ�����д��NH4Cl����ʯ�ҷ�Ӧ�Ļ�ѧ����ʽ ��
��3�������Ƽ��Ӧ�ķ���ʽΪ ��
��4��Ϊʲô�����Ƽ����������ʳ��ˮ��ͨ�백������ͨ�������̼�������� (д����)��
��5�������Ƽ��Ʒ�����к���̼�����ơ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�������������m1��ʾ����ǰ������Ʒ��������m2��ʾ���Ⱥ���������������̼�����Ƶ����������ɱ�ʾΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ȼ���������ض��dz�����ˮ����������ͼΪ�Ʊ��Ȼ�������һ�������Ʊ�������صĹ������̡�
��ش��������⣺
��1���Ȼ����ж�����;���������ӷ���ʽ��ʾ������;��ԭ����
���Ȼ�������ˮ��______________________��
����FeCl3��Һ��32%��35%����ʴͭӡˢ��·��____________________________��
��2�����ռ�X�Ļ�ѧʽΪ ���� ��������Y�Ļ�ѧʽΪ________________��
��3�����������·�Ӧ�ٵ����ӷ���ʽΪ____________________________________��
��4�����̢ڽ������Һ�����Сʱ�����ã����˻�ôֲ�Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ
2KOH��Na2FeO4��K2FeO4��2NaOH������ݸ��ֽⷴӦԭ��������Ӧ������ԭ��_________��
��5��K2FeO4��ˮ��Һ��������Ӧ��4FeO42-+10H2O 4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ���
4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ���
| A��H2O | B��ϡKOH��Һ������� | C��NH4Cl��Һ������� | D��Fe(NO3)3��Һ������� |
 CrO42-��Fe(OH)3����OH-
CrO42-��Fe(OH)3����OH- Cr2O72-��H2O
Cr2O72-��H2O 2Cr3����6Fe3����7H2O
2Cr3����6Fe3����7H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013��1��26��15ʱ�����й��������;���������ˣ�20�ɹ����䡣��־���ˣ�20��Բ���ɹ�����ش��������⣺
��.�ˣ�20���ݽṹ������Ҫ��ͬ��ʹ�õIJ���Ҳ��ͬ������Ϊ���в����������ˣ�20���죬�������� (�����)��
| A�����ô��������ˣ�20������ |
| B����þ���Ͻ������ˣ�20���� |
| C���������������ˣ�20������·�ĵ��� |
| D������ͨ�������ˣ�20���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������������һ�ֳ��õĽ������洦������������ʹ���ı�������һ�����ܵ�����Ĥ��������Ĥ������ϡ���ᡣ
ij��ѧ�о�С����ʵ�����а����в���ģ�������桰�ۻ����IJ������̡�
(1)����ʵ���õ���Һ��Ҫ����200 mL�ܶ�Ϊ1.2 g/cm3��������������Ϊ16%��NaOH��Һ����Ҫ��ȡ________g NaOH���塣
(2)����Ƭ�����ȵ�16%��NaOH��Һ��Լ���������ϴȥ���ۣ���ȥ���������Ĥ��ȡ����ˮ��ϴ��д����ȥ����Ĥ�����ӷ���ʽ��________��
(3)����ͼ��װ����������ͨ����K��ͨ��Լ25 min�������������������������������塣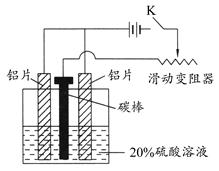
д���ù����еĵ缫��Ӧʽ��
������_________________________________________��
������_________________________________________��
(4)�Ͽ���·��ȡ����Ƭ������������Ϊ1%��ϡ��ˮ�кͱ������Һ������ˮ��ϴ�ɾ���д���ù��̷�����Ӧ�����ӷ���ʽ��______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com