����Լռ����������71%����һ��Զδ��ȫ�����ľ�ѧ��Դ���⣬��ˮˮ��Դ�����úͺ�ˮ��ѧ��Դ�����þ��зdz�������ǰ�����ش��������⣺
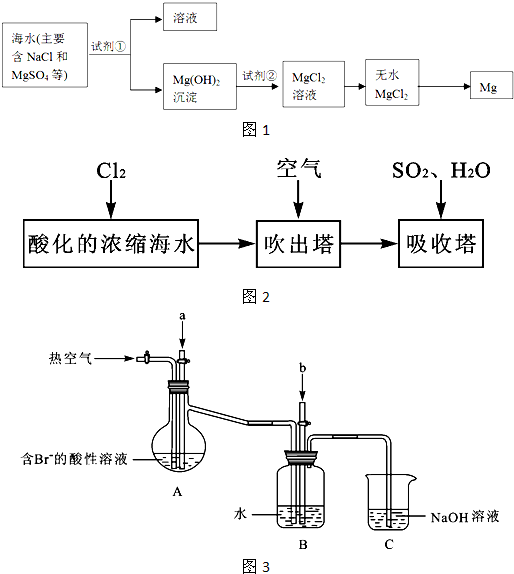
��1����ˮ������������������ͼ�Ǻ�ˮ����ԭ��ʾ��ͼ�������ص����豸�ṹ����������ˮ�����ã���Ҳ�������Ե�ȱ�ݣ�

1����Ϊ�����к�ˮ��������Ҫȱ����
���Ĵ������������ɱ�̫��
���Ĵ������������ɱ�̫��
��
����ʡij�غ���������������˽�һ�����ͺ�ˮ����������Ϊ�˷�����ˮ������ȱ�ݣ�����Ϊ�õ������һ������������
���÷��ܻ�̫����
���÷��ܻ�̫����
��
��2����ˮ������õ��ĵ�ˮӦ���м�����������ܵõ���ȫ��������������Ҫ���ˮ��ʹ�����ӽ�����֬��ˮ�е����ӽ��н����dz��õ�ˮ�����������۱�ϩ������һ�����ӽ�����֬����֪��ϩ���ƵĻ�ѧʽΪCH
2=CH-COONa��д�����ɾ۱�ϩ���ƵĻ�ѧ���̣�
nCH
2=CH-COONa

nCH
2=CH-COONa

��
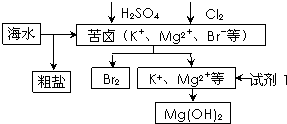
��3���Ӻ�ˮ�п��Ի�ȡʳ�Ρ�þ���ء��弰�仯����Ʒ��������������Ŀǰ�Ӻ�ˮ����ȡ��ij��÷����������������£�

��ʵ�����д����ᴿ�IJ�����
�ܽ⣬���ˣ������ᾧ
�ܽ⣬���ˣ������ᾧ
���ڷ�Ӧ���еõ�����Һ��ͨ�������ˮ������������
����������
����������
��
�ڷ�Ӧ�����ӷ���ʽ�ֱ�Ϊ
2Cl
-+2H
2O
2OH
-+Cl
2��+H
2����Cl
2+2Br
-=Br
2+2Cl
-��SO
2+Br
2+2H
2O=4H
++2Br
-+SO
42-2Cl
-+2H
2O
2OH
-+Cl
2��+H
2����Cl
2+2Br
-=Br
2+2Cl
-��SO
2+Br
2+2H
2O=4H
++2Br
-+SO
42-��
���ڷ�Ӧ�����ı����896m
3SO
2ʱ����ת��
80000
80000
mol���ӣ�
��4���������������߷������������̵�������
�����嵥��
�����嵥��
��










 ��2012?������һģ������Լռ����������71%��������п������õIJ���������ͼ��ʾ������˵������ȷ���ǣ�������
��2012?������һģ������Լռ����������71%��������п������õIJ���������ͼ��ʾ������˵������ȷ���ǣ�������