
����������һ���ķ����,��ijЩ������ˮ��Ӧ������з����ͼʾ��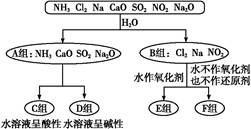
���������ѧ��֪ʶ,��Ҫ�����:
��1��������һ����������ֳ�A��B������ݣ����� ��
��2��F�������ʳ���Cl2��������������ѧʽ����
��3��A���е�CaO��������ʳƷ��װ���еĸ����,CaO��������������Ϊ�����������������գ���
�ٽ���������ڼ���������ۼ�ܼ��Ը����
CaO����������������� ���û�ѧ����ʽ��ʾ����
��4��D����NH3��ˮ��Һ��������,�õ��뷽��ʽ��ʾ��������Ե�ԭ��:�� ��
��5����Al3+�Ʊ�Al��OH��3,��ò�ѡ��D���е�NaOH��Һ,˵������:�� ��
 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�п�Ժ��ѧ�����Ƶľ�����ϡ��������������������ں˴Ź�����Ӱ��ҽҩ���й㷺��;���������̵IJ�����������ͼ��ʾ��
FeCl3��6H2O  FeOOH
FeOOH  ��������������
��������������
�������������������
| A�����������������ɷ�ɢ��ˮ�У�����FeCCl3��Һ�ķ�ɢ��ֱ���൱ |
| B�������������������д��ԣ�����Ϊҩ�������������Ƽ��� |
| C���ڷ�Ӧ���л����������ÿ����Ǵٽ��Ȼ���ˮ�� |
| D����Ӧ�ڵĻ�ѧ����ʽ��6FeOOH+CO=2Fe3O4+3H2OʮCO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�������أ�K2FeO4����һ�ּ�������������������һ������Ͷ��ˮ���������������������£�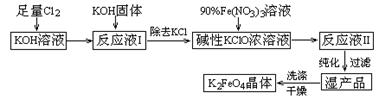
��֪���� 2KOH + Cl2 =" KCl" + KClO + H2O���������¶Ƚϵͣ�
�� 6KOH + 3Cl2 =" 5KCl" + KClO3 + 3H2O���������¶Ƚϸߣ�
�� 2Fe(NO3)3 + 2KClO + 10KOH = 2K2FeO4 + 6KNO3 + 3KCl + 5H2O
�ش��������⣺
��1������������Ӧ�� ����¶Ƚϸߡ����¶Ƚϵ͡���������½��У�
��2��д����ҵ����ȡCl2�Ļ�ѧ����ʽ ��
��3��K2FeO4����Ϊ���Ͷ��ˮ��������ԭ�� ��
��4����MnO2 �� Zn������ƣ�K2FeO4 �� ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ�������ϣ���缫��ӦʽΪ________���õ���ܷ�Ӧ�����ӷ���ʽΪ_____��
��5���ڡ���ӦҺI ���м�KOH�����Ŀ���Ǣ� ���� ��
��6���ӡ���ӦҺII ���з����K2FeO4����Ʒ�� ��д��ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������г��õ�����������������ᡱָ���ᡢ��������ᣬ�����ָ�ռ�ʹ��
��1�������ʵķ���Ƕȿ�����ǡ����һ�������� �������ʵĻ�ѧʽ����
��2�������ᡱ�롰���֮��ķ�Ӧ�����û�ѧ����ʽ��ʾ�������������ʱ�����������ӷ���ʽ��ʾȴֻ����������д�����������ӷ���ʽ���������ʱ�� �� ��
��3�������ᡱ�������ܽ�����ͽ�����������п�״�����ڳ���ʱ��ȫ����������Ũ������� ��
| A��Au | B��Cu | C��Al | D��Fe |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��B��C��DΪ���壬����AΪ����ɫ��D��������ˮ���γɵ���Һ��
ʹ��̪��죬����֮���ת����ϵ����ͼ����ʾ��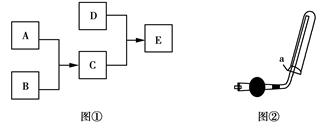
��1��������B��ȼ���ѵ�������ʢ������A�ļ���ƿ����Ӧ�����е�ʵ�������� ������ţ�
�ٷ��� �ڻ���ɫ��ȥ ��ƿ���а��� ��ƿ���а���
�ݰ���ȼ�գ�������ɫ���� �ް���ȼ�գ�������ɫ����
��2��ʵ������D�Ļ�ѧ����ʽΪ ��
��3��ʵ���ҿ�������ͼ����ʾװ���ռ�D������������ȷ���� ������ţ���
��D���岻������ˮ���ռ�
�ڸ������ʢ�м�ʯ��
��ͼ���е�aΪ���ţ��������Ƿ�ֹ�����ݳ�
��4������D�������Ļ�ѧ����ʽΪ ��
��5������E�Ļ�ѧʽ�� ��E���������ʷ��������������������� ������ţ���
�ٵ���ʣ��ڻ�����ۻ����ܴ�����ݷǵ���ʣ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Fe(OH)3����������������ҪӦ�ã�����FeCl3�ͷ�ˮ��Ӧ�Ʊ���Fe(OH)3�����г�����FeCl3��HCl����֪���岻������Ĥ����С���Ӻ����ӿ�������Ĥ���Իش������й����⣺
(1)ʵ������ȡFe(OH)3����ķ�����________����________������ȥ�����еĻ��������________����֤�������Ѿ��Ƴɡ�
(2)������Һ�д���Fe3����H���ķ�����__________________________________________
(3)��ȥ�����л��е�FeCl3��HCl�ķ����ǣ�_________________________________��
(4)�����ʵ��ķ���֤�������Cl���Ѿ����룿___________________________��
(5)����Fe(OH)3�����FeCl3��Һ��ķ�����_________________________________��
(6)��������(Na2FeO4)�������Ա�KMnO4��ǿ������һ�ֱ��ܹ�ע�����;�ˮ������ָ��Na2FeO4��ˮ��ԭ������˵���þ�ˮ���к���Խ��_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪһ��������ͼ����С����ͼ���������ҷֱ�д��H2��CO2��Na2O��NaCl��FeCl3�������ʣ�ͼ���������������ʾ��ɹ���Ϊһ�࣬�ཻ����������A��B��C��DΪ����Ӧ�ķ������ݴ��š�
��ش��������⣺
(1)�뽫�������ݴ���������Ӧ��������
( )�������ʶ����ǵ����
( )�������ʶ����ƵĻ�����
( )�������ʶ���������
( )�������ʶ�����
(2)�ýྻ���ձ�ȡ��������ˮ���þƾ��Ƽ��������ڣ����ձ�����μ���
1 mol��L-1��ͼ��һ�ֻ������ˮ��Һ�����Ƶ�һ�ֺ��ɫ���塣
�ٷ�Ӧ�Ļ�ѧ����ʽΪ��________________________________________________��
������ýϼķ����жϽ����Ƿ��Ʊ��ɹ���
___________________________________________________________
����ý�������μ������ᣬ�����һϵ�б仯��
a���ȳ��ֺ��ɫ������ԭ����___________________________________________
b�������ɫ�����ܽ⣬�˷�Ӧ�����ӷ���ʽ��____________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ʮ�����ʣ���H2 ���� ��CaO ��CO2 ��H2SO4 ��Ba(OH)2 �ߺ��ɫ����������Һ�� �ఱˮ ��ϡ���� ��Al2(SO4)3
��1�����������ʰ����ʵķ������д����Ŀհ״��������ʱ�ţ���
| ����� | �������� | ������ | ��Һ | ���� | ����� |
| ���ڸ�������� | | | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й���������ȷ����
| A��������ˮ�ĵ����һ����������� |
| B��ǿ�������ˮ��Һ�еĵ�������Dz������ |
| C��������ˮ�ĵ����һ����ǿ����� |
| D��ǿ����ʵ�ˮ��Һ��������һ�����������ˮ��Һ�ĵ�������ǿ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com