
Fe(OH)3����������������ҪӦ�ã�����FeCl3�ͷ�ˮ��Ӧ�Ʊ���Fe(OH)3�����г�����FeCl3��HCl����֪���岻������Ĥ����С���Ӻ����ӿ�������Ĥ���Իش������й����⣺
(1)ʵ������ȡFe(OH)3����ķ�����________����________������ȥ�����еĻ��������________����֤�������Ѿ��Ƴɡ�
(2)������Һ�д���Fe3����H���ķ�����__________________________________________
(3)��ȥ�����л��е�FeCl3��HCl�ķ����ǣ�_________________________________��
(4)�����ʵ��ķ���֤�������Cl���Ѿ����룿___________________________��
(5)����Fe(OH)3�����FeCl3��Һ��ķ�����_________________________________��
(6)��������(Na2FeO4)�������Ա�KMnO4��ǿ������һ�ֱ��ܹ�ע�����;�ˮ������ָ��Na2FeO4��ˮ��ԭ������˵���þ�ˮ���к���Խ��_______________________________________��
(1)�ڷ��ڵ�����ˮ�еμӱ���FeCl3��Һ������Һ�ʺ��ɫʱ��ֹͣ���ȣ����Ƶý��塡���ˡ������
(2)ȡ������Һ���μ�KSCN��Һ�����ɫ˵����Fe3������ȡ������Һ���μ���ɫʯ����Һ�����ɫ˵����H��
(3)������װ���Ĥ�У�Ȼ����������ˮ��(����)
(4)ȡ��Ĥ�����һ�ε���Һ�������Թ��У����������ữ��AgNO3��Һ��������������֤�������Ѿ�����
(5)�۲���ɫ
(6)Na2FeO4�к�ǿ�������ԣ�����Ч��ɱ��ˮ�е�ϸ���Ͳ������仹ԭ����Fe3���ܷ���ˮ�ⷴӦ����Fe(OH)3���壬��������ˮ�е����ʡ�Na2FeO4�����������;��������У��������κζ������к�������
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������ͨ��������Һʱ���Ӳ���۲쵽һ�������ġ�ͨ·����˵��������Һ��
| A������ | B������Һ | C����Һ | D������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��17�֣��������������ʣ��پƾ�����ͭ���������������ܰ����������ǡ� �����ᡢ��̼�����ơ������ᡢ�����⡢��Al2(SO4)3���������ʵ������д���пհ�
��1������ǿ����ʵ��У� ��
��2��Һ̬ʱ�ܵ�����Ϊ�����仯���У� ��
��3������ˮ��Һ�ĵ��뷽��ʽΪ ��
��A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʡ�����֮�������µķ�Ӧ��ϵ��
��1����A�ǵ���ɫ���壬C��D���������C������������Ҫ���ʡ�����C���ʿɵõ��м�ֵ�Ļ�ѧƷ��д���û�ѧƷ�е�1�����1���ε������� ������������ ��������
��2����B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ��B��C��һ�������·�Ӧ���ɵ�A�Ǵ�����Ҫ�ɷ֣�д���÷�Ӧ�Ļ�ѧ����ʽ������������ �� ������ ����������
��3����D���ʾ������ԣ��ڢ۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧ��ͨ�������һ����������ЧӦ����Ҫ���塣�жϵ���A��Ԫ�������ڱ��е�λ����___ _____��д�ܷ�Ӧ���ӷ�����������
��4����A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A���ڢݷ�Ӧ���õ�ͬһ�ַǽ������ʡ�C����Һ����ʴ��ӡˢͭ��·�壬д�÷�Ӧ�����ӷ���ʽ����������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ�У�NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺
��1��NH4Al(SO4)2������ˮ������������ (�ñ�Ҫ�Ļ�ѧ������������˵��)��
��2����ͬ�����£�0��1 mol��L��1NH4Al(SO4)2��c(NH4+) (����ڡ��������ڡ���С�ڡ�)0��1 mol��L��1NH4HSO4��c(NH4+)��
��3��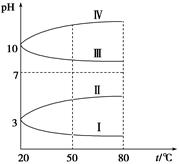
��ͼ��0��1 mol��L��1�������Һ��pH���¶ȱ仯��ͼ��
�����з���0��1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯�������� (��д��ĸ)������pH���¶ȱ仯��ԭ���� ��
��20 ��ʱ��0��1 mol��L��1NH4Al(SO4)2��2c(SO42-)��c(NH4+)��3c(Al3��)�� ��
��4������ʱ����100 mL 0��1 mol��L��1NH4HSO4��Һ�еμ�0��1 mol��L��1NaOH��Һ���õ���ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��
�Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶������� ����b�㣬��Һ�и�����Ũ���ɴ�С������˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������һ���ķ����,��ijЩ������ˮ��Ӧ������з����ͼʾ��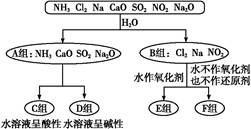
���������ѧ��֪ʶ,��Ҫ�����:
��1��������һ����������ֳ�A��B������ݣ����� ��
��2��F�������ʳ���Cl2��������������ѧʽ����
��3��A���е�CaO��������ʳƷ��װ���еĸ����,CaO��������������Ϊ�����������������գ���
�ٽ���������ڼ���������ۼ�ܼ��Ը����
CaO����������������� ���û�ѧ����ʽ��ʾ����
��4��D����NH3��ˮ��Һ��������,�õ��뷽��ʽ��ʾ��������Ե�ԭ��:�� ��
��5����Al3+�Ʊ�Al��OH��3,��ò�ѡ��D���е�NaOH��Һ,˵������:�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��ֻ��һ���Լ����ɳ�ȥ�������ʺͼ������ʡ��������ڿո�
| ��� | ���� | �Լ����ƻ�ѧʽ |
| �� | �����ʣ�NaHCO3��Һ��Na2CO3�� | |
| �� | �����ʣ�SiO2��CaCO3�� | |
| �� | �����ʣ�FeCl2��Һ��FeCl3�� | |
| �� | ����Na2CO3 Na2SiO3 Na2SO3��Һ | |
| �� | ���𣺣�NH4��2SO4 NH4C1 Na2SO4��Һ | |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������̽��Ҫ�õ����ѧ֪ʶ��
(1)�±��г������������Ħ���������ڱ�����д����Ħ�����������������
| ���� | �������ͯ���� | �������������� | �л������� |
| Ħ���� | �������� | ̼��� | �������� |
| Ħ�������������(ָ�ᡢ��Ρ������������������) | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���и������ʣ��� O2��O3�� �� 12C��14C���� CH3CH2CH2CH3��(CH3)2CHCH3��������Ͷ��飻�� CH3CH2CH2CH(C2H5)CH3�� CH3CH2CH2CH(CH3)C2H5��
��Ϊͬϵ����� ����Ϊͬ���칹����� ����Ϊͬλ�ص��� ����Ϊͬ����������� ����ͬһ���ʵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���з�Ӧ�����ӷ���ʽ��ȷ����
| A��̼�����ϡ���ᷴӦ�� CaCO3+2H+�� Ca2++ CO2��+H2O |
| B����������ˮ��Ӧ�� 2Na +2H2O ��2Na+ +2OH��+ H2�� |
| C��������ϡ�����У� 2Fe��6H+�� 2Fe3+��3H2�� |
| D������������Һ�еμ�ϡ���Ba2+ + SO42����BaSO4�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com