

���� ��1���廯��ͭ�ܱ���������������Ҫ�ų������ĸ��ţ�
��2����������ƿ��ͭ���ӱ���������ԭ����ͭ���ӣ��������ӷ�Ӧ����CuBr������
�ڿ��Ʒ�Ӧ��60����У�������60���ˮԡ���ȣ�
��45gCuSO4•5H2OΪ0.18mol��19gNaBrΪ0.184mol������NaBr�Թ��������Ե���Һ�е�ͭ����������ʱ��Ӧ����ɣ�
��3���廯��ͭ�����ֽ⣻
��4���廯��ͭ�ڿ����л�������������ϴ�Ӽ��衰����SO2�����Է�ֹCuBr���������ܼ��������ѿ��Գ�ȥ�����Ҵ�����ʹ������ٸ��
��5���ձ��е�����Һ��Ҫ��Na2SO3��NaHSO3�ȣ���ȡ�ϴ�����Na2SO3•7H2O���壬�������ձ��м���ͨ��SO2�����ͣ���Na2SO3����NaHSO3��������Ԫ���غ��֪����ʱ��Һ��NaHSO3�����ʵ���Ϊ0.5mol��Ȼ�����ձ��м���100g 20%��NaOH��Һ��ʹNaHSO3ǡ����ȫ��Ӧ����Na2SO3����������ά����C��Һ����������������Ũ������ȴ�ᾧ�����ˣ����Ҵ�ϴ��2��3�Σ���ȥ������������ʣ�����ո������и���ݴ˴��⣻
��� �⣺��1���廯��ͭ�ܱ����������������ö�������ԭͭ���������廯��ͭҪ�ų������ĸ��ţ�����ͨ����еķ�����ȥ����ˮ�е�O2��
�ʴ�Ϊ��O2��
��2����������ƿ��ͭ���ӱ���������ԭ����ͭ���ӣ��������ӷ�Ӧ����CuBr��������Ӧ�����ӷ���ʽΪ2Cu2++2Br-+SO2+2H2O=2CuBr��+SO42-+4H+��
�ڿ��Ʒ�Ӧ��60����У�������60���ˮԡ���ȣ�
��45gCuSO4•5H2OΪ0.18mol��19gNaBrΪ0.184mol������NaBr�Թ��������Ե���Һ�е�ͭ����������ʱ��Ӧ����ɣ�����˵����Ӧ����ɵ���������Һ��ɫ��ȫ��ȥ��
�ʴ�Ϊ��2Cu2++2Br-+SO2+2H2O=2CuBr��+SO42-+4H+��60��ˮԡ���ȣ���Һ��ɫ��ȫ��ȥ��
��3���廯��ͭ�����ֽ⣬���Բ���2������Ҫ�ܹ⣬��ֹCuBr����ֽ⣬
�ʴ�Ϊ����ֹCuBr����ֽ⣻
��4���ڿ����л�����������������ϴ�Ӽ��衰����SO2�����Է�ֹCuBr������������ܼ��������ѿ��Գ�ȥ�����Ҵ�����ʹ������ٸ��
�ʴ�Ϊ����ֹCuBr����������ȥ�����Ҵ�����ʹ������ٸ��
��5���ձ��е�����Һ��Ҫ��Na2SO3��NaHSO3�ȣ���ȡ�ϴ�����Na2SO3•7H2O���壬�������ձ��м���ͨ��SO2�����ͣ���Na2SO3����NaHSO3��������Ԫ���غ��֪����ʱ��Һ��NaHSO3�����ʵ���Ϊ0.5mol��Ȼ�����ձ��м���100g 20%��NaOH��Һ��ʹNaHSO3ǡ����ȫ��Ӧ����Na2SO3����������ά����C��Һ����������������Ũ������ȴ�ᾧ�����ˣ����Ҵ�ϴ��2��3�Σ���ȥ������������ʣ�����ո������и��
�ʴ�Ϊ�������ձ��м���ͨ��SO2�����ͣ���Ȼ�����ձ��м���100g 20%��NaOH��Һ���ܹ��ˣ����Ҵ�ϴ��2��3�Σ�
���� ���⿼���������Ʊ��������������̣��漰�Բ����ķ������ۡ����ʵķ����ᴿ��ʵ�鷽��ʵ�ʵȣ���5��Ϊ�״��㣬ѧ�������Լ�������������Һ���������Ǹ߿��ȵ����ͣ���Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 25��ʱ��pH=3��CH3COOH��Һ��ˮϡ��10����pH=4 | |
| B�� | 25��ʱ��pH=11��NaOH��Һ��ˮϡ��100����pH=9 | |
| C�� | 25��ʱ��pH=3��������pH=11�İ�ˮ�������Ϻ�pH=7 | |
| D�� | 90��ʱ����ˮ��pH=6.2�����Լ��ȿ���ʹˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ᡢ���ͭ�̡���ʯ�ҷֱ������ᡢ��Ρ������� | |
| B�� | HClO��H2SO4��Ũ����HNO3������ǿ�����ԣ������������� | |
| C�� | Al��Al2O3��Al��OH��3�����������ᷴӦ�������������Ʒ�Ӧ�����������Ի����� | |
| D�� | H2SO4��NaOH��AlCl3��Ϊǿ����ʣ����������ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�22.4L��NO2��CO2��������к��е���ԭ����Ϊ4NA | |
| B�� | �ܱ������У�46gNO2��N2O4�Ļ�������������Ӹ���ΪNA | |
| C�� | ���³�ѹ�£�22.4L��Һ̬ˮ����2.24��10-8NA��OH- | |
| D�� | �����£�16.8 g Fe������ˮ������ȫ��Ӧʧȥ0.8NA������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2CH4��g��+4O2��g���T2CO2��g��+4H2O��l����H=+890kJ•mol-1 | |
| B�� | CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=+890kJ•mol-1 | |
| C�� | CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=890kJ•mol-1 | |
| D�� | $\frac{1}{2}$ CH4��g��+O2��g���T$\frac{1}{2}$CO2��g��+H2O��l����H=-445kJ•mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
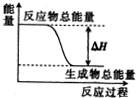
| A�� | ���ȵ�̼�������̼��Ӧ | |
| B�� | ̼��Ƶķֽ� | |
| C�� | Ba��OH��2•8H2O�����NH4Cl������ | |
| D�� | ��������������ת���ɶ�����̼��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����̼���仯��������ʣ��������С�⣺
����̼���仯��������ʣ��������С�⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 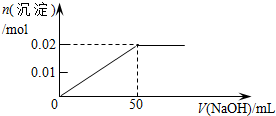 | B�� |  | ||
| C�� | 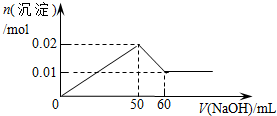 | D�� |  |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com