


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
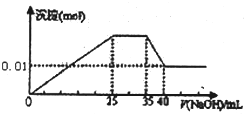
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
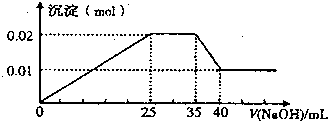
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| (m-132n) |
| 42 |
| (m-132n) |
| 42 |
�鿴�𰸺ͽ���>>
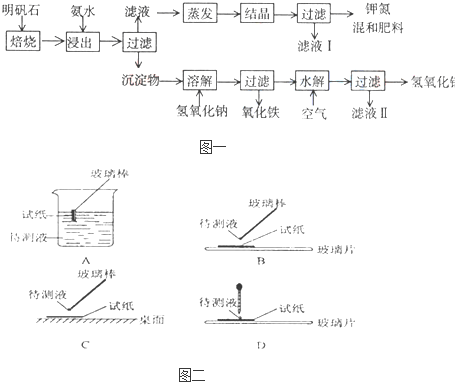
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��һδ֪����ɫ��Һ��ֻ���ܺ������������е������֣�H+��NH+4��K+��Mg2+��Cu2+��Al3+��NO-3��CO2-3��SO2-4����ȡ����100mL��Һ��������ʵ��
��һδ֪����ɫ��Һ��ֻ���ܺ������������е������֣�H+��NH+4��K+��Mg2+��Cu2+��Al3+��NO-3��CO2-3��SO2-4����ȡ����100mL��Һ��������ʵ��| A��ԭ��Һһ��������H+��Cu2+��CO2-3 | B������ȷ��ԭ��Һ�Ƿ���K+��NO-3 | C��ԭ��Һȷ����Mg2+��Al3+��NH+4����n��Mg2+����n��Al3+����n��NH+4��=1��1��2 | D��ʵ�����ӵ�NaOH��Ũ��Ϊ2 mol?L-1 |
�鿴�𰸺ͽ���>>
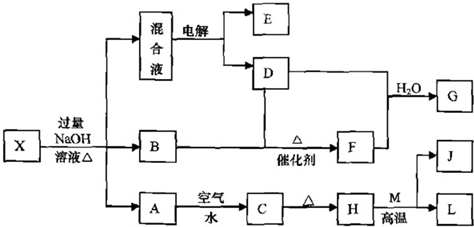
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
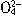 ��N
��N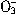 ��I-��S2-��H+��K+�������е����ֻ���֡���������ʵ�飺��ȡ������Һ����������������ų�������ȡԭ��Һ����Na2SO3��Һ��Ҳ������ų������а�ɫ�������ɣ��ټ������������ȫ��ʧ������ȡԭ��Һ����AgNO3��Һ�������ɡ��ɴ��жϣ���1��ԭ��Һ��һ�����е�������__________________����2��һ��������������________________����3�����ܺ��е�������______________��
��I-��S2-��H+��K+�������е����ֻ���֡���������ʵ�飺��ȡ������Һ����������������ų�������ȡԭ��Һ����Na2SO3��Һ��Ҳ������ų������а�ɫ�������ɣ��ټ������������ȫ��ʧ������ȡԭ��Һ����AgNO3��Һ�������ɡ��ɴ��жϣ���1��ԭ��Һ��һ�����е�������__________________����2��һ��������������________________����3�����ܺ��е�������______________���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com