
��ÿ��2�֣���10�֣����꣬����ԭ�ͼ۸������ǣ���ʹ���������Ӵ����������������оƾ������ѽ���ʵ�û��Ρ�
(1)�Ҵ���ͭ�����������������£����Ա������е�����������X��X�ɷ���������Ӧ����д��X��������Һ�����ķ�Ӧ����ʽ(������巴Ӧ����)��______________________________________��
(2)�Ҵ������������ظ������Һ��Ӧ����ֱ��������Y��Y���й����ŵ�������________����Ũ��������¼��ȣ��Ҵ���Y��Ӧ������һ������ζ������W����ѧ����ʽΪ________________________________________��
(3)������ƿ��ɫҺ�壬�ֱ�ʢ��Y��W��ֻ��һ���Լ��Ϳ��Լ��𣬸��Լ�������____________________________________��
(4)�ִ�ʯ�ͻ�������������������ϩ�ܱ������������ɻ�������( )�÷�Ӧ��ԭ��������Ϊ100%����Ӧ�Ļ�ѧ����ʽΪ___________��
)�÷�Ӧ��ԭ��������Ϊ100%����Ӧ�Ļ�ѧ����ʽΪ___________��
(1)CH3CHO��2Ag(NH3)2OH CH3COONH4��2Ag����3NH3��H2O
CH3COONH4��2Ag����3NH3��H2O
(2)�Ȼ���CH3COOH��HOCH2CH3 CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O
(3)ʯ��(������������)��
(4)2CH2===CH2��O2 2
2
���������������1���Ҵ���ͭ�����������������£����Ա������е�������������ȩ����ȩ��������Һ����������Ӧ����������李�����������ˮ����ѧ����ʽΪCH3CHO��2Ag(NH3)2OH CH3COONH4��2Ag����3NH3��H2O��
CH3COONH4��2Ag����3NH3��H2O��
��2���Ҵ������������ظ������Һ��Ӧ����ֱ�����������ᣬ����Y�����ᣬ�����Ȼ������ţ��Ҵ������ᷢ��������Ӧ����ѧ����ʽΪCH3COOH��HOCH2CH3 CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O
��3�����ݣ�2����֪Y�����ᣬW���������������������ԣ���ʹ��ɫʯ����Һ��죬���Լ������ᡢ�����������Լ���ʯ����Һ��
��4��������Ŀ������Ϣ����ϩ��������Ӧ��ԭ��������100%��˵����ϩ��������Ӧֻ���ɻ������飬��ѧ����ʽΪ2CH2===CH2��O2 2
2
���㣺�����Ҵ��Ļ�ѧ���ʣ����ʵ��жϣ���ѧ����ʽ����д



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ֽͨ�ŵ���Ҫ�ɷ�����ά�أ������ڵ�ֽ�������У�������ֽ����Ϳ�������Ĺ��գ�����������ף���ֹī����ɢ����ش��������⣺
(1)���Ƿ���ֽ�Żᷢ�����Ը�ʴ����ࡢ����������вֽ������ı��档���������飬�������Ը�ʴ��Ҫ����ֽ��Ϳ�������Ĺ����йأ����еĻ�ѧԭ����
____________________________________________________________________________. (2)Ϊ�˷�ֹֽ�ŵ����Ը�ʴ������ֽ���м���̼��Ƶ����Ӽ����ù���ԭ���Ļ�ѧ����ʽΪ___________________��
Ϊ�˱�����Щֽ��������˽����ȡ���д�ʩ��
(3)����������Һ����ϡ����������Һ��ˮ�ȣ�����������������Ҫ������
___________________________________________________________________________��
(4)����Zn(C2H5)2��Zn(C2H5)2������ˮ��Ӧ��������п�����顣�û�ѧ(����)����ʽ��ʾ�÷�����������п����ֹ���Ը�ʴ��ԭ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���й����������ʵ������У���ȷ���ǣ� ��
| A���������Ǵ�������ˮ��Һ |
| B�������������Ʒ�Ӧ�ų����� |
| C����������Ա�̼��ǿ�����������Ը�̼������Һ��Ӧ����CO2���� |
| D����������к���̼��˫������������ʹ��ˮ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼAװ���Ʊ�����������
��1����ʵ����������ͺ�18O���Ҵ����ã��÷�Ӧ�Ļ�ѧ����ʽ�ǣ�
��̲IJ��õ�ʵ��װ�ò�ͬ����װ���в��������θ���ܣ��������� ��
��2��Ϊ��֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý���������4��ʵ�飮ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܢ����Լ� | �Թܢ����Լ� | �л���ĺ��/cm |
| A | 2mL�Ҵ���1mL���ᡢ 1mL18mol?L-1 Ũ���� | ����Na2CO3��Һ | 3.0 |
| B | 2mL�Ҵ���1mL���� | 0.1 | |
| C | 2mL�Ҵ���1mL���ᡢ 3mL 2mol?L-1H2SO4 | 0.6 | |
| D | 2mL�Ҵ���1mL���ᡢ���� | 0.6 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��11��E�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־�����ܽ�����ͼ��ʾ�ķ�Ӧ��
��1��д��A�Ĺ�����������������������
��2�����з�Ӧ�Ļ�ѧ����ʽ
��Ӧ�٣����������������������� ������ �� �����ķ�Ӧ��������������������
��Ӧ�ڣ����������������������������������������������ķ�Ӧ���������� ��������
��Ӧ�ܣ����������������������������������������������ķ�Ӧ���������� ��������
��3���Ƚϣ���Ӧ�پ��ҳ̶� �����= ���ƺ�ˮ��Ӧ�ľ��ҳ̶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣���֪±������R-X���ڼ��������¿�ˮ��õ�����R-OH�����磺CH3CH2-X+H2O  CH3CH2-OH+HR����������ת����ϵ:
CH3CH2-OH+HR����������ת����ϵ: 
�ش��������⣺
��1����Ӧ1���Լ�������Ϊ __________��X�Ľṹ��ʽΪ______��Y�Ľṹ��ʽΪ______��
��2��д����Ӧ3�ķ���ʽ___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�ij��ѧС���Ա�����Ϊԭ�ϣ���ȡ�������������֪�й����ʵķе������
| ���� | �״� | ������ | ��������� |
| �е�/�� | 64.7 | 249 | 199.6 |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
һ���л���Ľṹ��ʽΪ��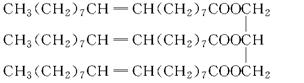
�Իش��������⣺
(1)�û������������________��
A��ϩ�� B���� C����֬ D���߷��ӻ�����
(2)�û�������ܶ�________��
A����ˮ�� B����ˮС
(3)�û����ﳣ���µ�״̬Ϊ________��
A��Һ�塡��B�����塡��C������
(4)��������ܷ�Ӧ����________��
A��NaOH��Һ B����ˮ
C���Ҵ������� D�����ᡡ���� E��H2
д�����л�����ѡ���Ļ����ﷴӦ�Ļ�ѧ����ʽ(��дһ��)��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�۲���ͼ�����ʷ��ӵı���ģ�ͼ���仯���ش��������⡣
(1)A���Ҵ�����B��������____________��
C�Ľṹ��ʽ��____________________________��
(2)��ͼ��ʾ��ѧ����ʽ�еġ���Ӧ��������___________________��
(3)��Ӧ����ֻ���Ҵ������е���ԭ����18O���������к�������ԭ�ӵ�������(д��������)______________ _��
(4)��B�ķ����У�����������Ϊ8��ԭ�ӹ���________����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com