
����Ŀ��[��ѧѡ��3�����ʽṹ�����ʡ�
�������ʯ�е�һ�֣�����Ҫ�ɷ�Ϊ��������-NaAI(Si2O6)��������Cr��Ni��Mn��Mg��Fe��Ԫ�ء��ش��������⣺
(l)��̬Crԭ�ӵĵ����Ų�ʽΪ____��Feλ��Ԫ�����ڱ���___ ����
(2)�������Ҫ�ɷֹ���ê�Ʊ�ʾΪ������Ļ�ѧʽΪ____����������Ԫ�ص�һ��������С�����˳����____��
(3)�ƺ������ǵ�������Ԫ�أ���ԭ�ӵ�������������ͬ��Ϊʲô�����۷е�Զ���ڸƣ�____��
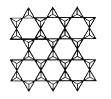
(4)�ڹ������д���![]() �ṹ��Ԫ������Siԭ�ӵ��ӻ��������Ϊ____�������������n��ʾ��
�ṹ��Ԫ������Siԭ�ӵ��ӻ��������Ϊ____�������������n��ʾ��![]() �ֱ���3��������������3��
�ֱ���3��������������3��![]() �γɲ�״�ṹʱ����ͼ��ʾ��������Si��Oԭ�ӵ���Ŀ֮��Ϊ____��
�γɲ�״�ṹʱ����ͼ��ʾ��������Si��Oԭ�ӵ���Ŀ֮��Ϊ____��
��������һ���Si��Al�滻���仯ѧʽΪ____��
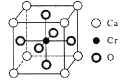
(5) Cr��Ca�����γ��־���������Եĸ�������������ṹ��ͼ��ʾ���þ���Ļ�ѧʽΪ____����Ca��O�ĺ˼����Ϊx nm����þ�����ܶ�Ϊ___ g/cm3��
���𰸡� [Ar] 3d54s1 d�� Na2O��Al2O3��4SiO2 Na��Al��Si��O Fe�ĺ˵�����ϴ˶Ե��ӵ������ϴ�Fe��ԭ�Ӱ뾶С��Ca��Fe�Ľ�����ǿ��Ca sp3 2�U5 [AlSiO5]![]() CaCrO3
CaCrO3 ![]()
��������(l) CrΪ24��Ԫ�أ����ݺ�������Ų����ɻ�̬Crԭ�ӵĵ����Ų�ʽΪ[Ar] 3d54s1��FeΪ26��Ԫ�أ�λ�ڵ������ڵ�VIII�壬Ҳ����Ԫ�����ڱ���d������Ϊ��[Ar] 3d54s1 ��d��
��2������������NaΪ+1���� AlΪ+3���� SiΪ+4�ۣ�OΪ-2�������Ա�ʾΪ������Ļ�ѧʽΪNa2O��Al2O3��4SiO2���ǽ�����Խǿ��һ������Խ��ͬһ���ڵ�Ԫ���������ҵ�һ���������������Na�� Al�� Si��O ����Ԫ�ص�һ��������С�����˳����Na��Al��Si��O ����Ϊ��Na2O��Al2O3��4SiO2 ��Na��Al��Si��O
��3���ƺ�������ͬһ���ڣ������ĺ˵�������ڸƣ����������ӵ���������ǿ��ʹ��������Խ����ԭ�Ӻˣ���������ԭ�Ӱ뾶С�ڸƣ���˽�����ǿ�ڸƣ����������۷е�Զ���ڸơ���Ϊ��Fe�ĺ˵�����ϴ˶Ե��ӵ������ϴ�Fe��ԭ�Ӱ뾶С��Ca��Fe�Ľ�����ǿ��Ca
(4) �ڹ������У��������SiO44-��Ϊ��������ṹ��ÿ��Si����Χ4��O�γ�4���Ҽ���Si�µ��Ӷԣ���������ԭ��Siԭ�Ӳ�ȡ��sp3�ӻ���ʽ��ÿ��������ͨ��������ԭ�������������������γɲ�״�ṹ�����ÿ���������й�ԭ������1����ԭ������1��3��![]() ��
��![]() ����Si��O��ԭ�Ӹ�����Ϊ2��5����ѧʽΪ(Si2nO5n)2n-��������һ���Si��Al�滻���仯ѧʽΪ
����Si��O��ԭ�Ӹ�����Ϊ2��5����ѧʽΪ(Si2nO5n)2n-��������һ���Si��Al�滻���仯ѧʽΪ![]() ��Ϊ��2��5��
����2��5��![]()
(5) ���ݾ����ṹͼ�;�̯����֪��������Oԭ����Ϊ��6=3��Caԭ����Ϊ��8=1��Crԭ����Ϊ1����ѧʽΪCaCrO3���辧���ı߳�Ϊacm������Ca��O�ĺ˼����Ϊxnm����2a2=4x2![]() ������a=
������a=![]() cm, CaCrO3��ʽ��Ϊ��140�����һ������������m=
cm, CaCrO3��ʽ��Ϊ��140�����һ������������m=![]() g�������������V=
g�������������V=![]() cm3�������þ�����ܶ���=
cm3�������þ�����ܶ���=![]() =
=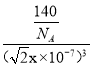 =
=![]() g/cm3������ CaCrO3��
g/cm3������ CaCrO3��![]()



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ڼ��������¿ɻ�ԭ����ͭ����������ˮ�����⣬����̼�������ij��ѧС��������ͼװ��̽���䷴Ӧ���

[��������]��CO����������Һ��Ӧ��CO��2[Ag(NH3)2]����2OH��===2Ag����2NH4+��CO32����2NH3��
��Cu2OΪ��ɫ������Ag+��Ӧ���ܷ�����Ӧ��Cu2O��2H��===Cu2+��Cu��H2O��
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
��2�������������װ�ô����ҵ�����˳��ΪA��__________________��(����ĸ���)
��3��ʵ���еμ�ϡ����IJ���Ϊ______________________________________________��
��4����֪��������к���CO����װ��C�пɹ۲쵽��������________________��װ��F������Ϊ_________________________________________��
��5������Ӧ������װ��D���Թ��й���ȫ����Ϊ��ɫ��
�����ʵ��֤����ɫ�����к���Cu2O��______________________________________________��
����֤����ɫ�������Ƿ���Cu����ͬѧ�������ʵ�飺��������ɫ�����м�������0.1mol��L1AgNO3��Һ��������Һ�������ݴ��жϺ�ɫ�����к���Cu����ͬѧ��Ϊ�÷�������������֤����ͬѧ�Ľ��ۣ������������¶Ա�ʵ�飬��ɱ������ݡ�
ʵ�鲽��(��Ҫ��д�������������) | Ԥ������ͽ��� |
__________________ | ���۲쵽��Һ����������֤����ɫ�����к���Cu�����۲쵽��Һ����������֤����ɫ�����к���Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������������ṹ��������ƽ�������νṹ��������(����)
A.CH3Cl������ͬ���칹��
B.CH2Cl2������ͬ���칹��
C.CHCl3������ͬ���칹��
D.�����ǷǼ��Է���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б�����Ԫ�����ڱ���һ���֣��밴Ҫ�����
��1��Ԫ���������γɵĻ������д��ڵĻ�ѧ������Ϊ__________________
��2��д�����٢ڢ��γɵ�һ��һԪ��ķ���ʽ_________________
��3��д�������γɵĵ��ʵĵ���ʽ_________________
��4���о�Ԫ�����γɵ�������һ����;________________��Ԫ���������ڱ��е�λ�� ____��
��������ŷḻ�ĺ�ˮ��Դ����ˮ��Ԫ���ݡ��������ĺ����ܷḻ��ij��ѧ��ȤС���Ƚ���ˮ������õ�ˮ���ٴ�ʣ���Ũ��ˮ��ͨ��һϵ�й�����ȡ������Ʒ����ش���������
�ش��������⣺
��5����ˮ�����ķ�����Ҫ��___________________________________(�����о�2��)
��6����������������������Ũ��ˮ�д���Br2�������£�Br2����ɫΪ___________________��
���������ô�����Һ���գ����������Ҫ��Ӧ��Br2+Na2CO3+H2O��NaBr+NaBrO3+ NaHCO3(δ��ƽ)��������1mol Br2ʱ��ת�Ƶĵ�����Ϊ________mol��
��7���Ӻ�ˮ�л��Ԫ���������Ļ������һ�ι���������ͼ��
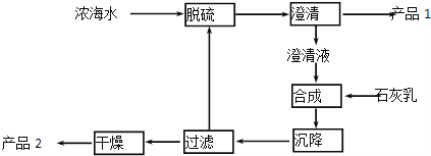
Ũ��ˮ����Ҫ�ɷ�����:
���� | Na+ | Mg2+ | Cl- | SO42- |
Ũ��/(g��L-1) | 63.7 | 28.8 | 144.6 | 46.4 |
�ù��չ����У���Ʒ1�Ļ�ѧʽΪ________________����Ʒ2ΪMg(OH)2����������Ũ��ˮ�еμ�NaOH��Һ����Mg2+ǡ����ȫ����ʱ��Һ��pHΪ_________��(��֪25��ʱKsp[Mg(OH)2]=1.0��10-13)
��8������MgCl2��6H2O�����Ʊ�MgCl2ʱ��ʵ����ȡ�óɹ��Ĺؼ�������������_________��
����ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪ______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO��SO2����Ҫ�Ĵ�����Ⱦ���壬���û�ѧ��Ӧԭ����������Ⱦ����Ҫ������
�����״����Բ���Ͳ������ʯ��ȼ�ϣ�������Դ���ţ�����CO���Ժϳɼ״���
��1����֪��CO(g)+1/2O2(g)�TCO2(g)��H1=-283.0kJ��mol��1
H2(g)+1/2O2(g)�TH2O(l)��H2=-285.8kJ��mol��1
CH3OH(g)+3/2O2(g)�TCO2(g)+2H2O(l)��H3=-764.6 kJ��mol��1
��д��CO��H2�ϳɼ״��������Ȼ�ѧ����ʽ____________________
��2��һ�������£����ܼ�ΪVL���ܱ������г���a molCO��2a molH2�ϳɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��

�ٸ÷�Ӧ��A���ƽ�ⳣ��K=_________________(��a��V��ʾ)
��������˵����Ӧ�ﵽƽ��״̬����_____
A.v(CO)=v(H2) B.���������ܶȲ���
C.��������ƽ����Է����������� D. c(CO)=c(H2)
��д��������v(CO)�������COת���ʵ�һ���ʩ_____________________________
����ijѧϰС����SO2Ϊԭ�ϣ����õ绯ѧ������ȡ���ᡣ
��3��ԭ���ԭ������С����Ƶ�ԭ��ʾ��ͼ������ͼ��д���õ�ظ����ĵ缫��Ӧʽ______��
��4�����ԭ������С����Na2SO3��Һ�������SO2�õ�NaHSO3��Һ��Ȼ�������Һ�Ƶ������ᡣԭ����ͼ��д����ʼ���ʱ�����ĵ缫��Ӧʽ________________��


��5����֪25��ʱ��Na2SO3��NaHSO3�γɵĻ����Һǡ�ó����ԣ���û����Һ�и�����Ũ�ȵĴ�С˳��Ϊ________________________________(��֪25��ʱ��H2SO3�ĵ���ƽ�ⳣ��Ka1=1��10-2��Ka2=1��10-7)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������7�ֻ�ѧ���ţ�![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]()
(1)��ʾ���صķ��Ź�______�֡�
(2)��Ϊͬλ�ص���______��______��
(3)��������ȣ������ܻ�Ϊͬλ�ص���______��______��
(4)��������ȣ�������������ȵ���______��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ں�����ʳ�����ڽ���������˵������ȷ���ǣ��� ����
A.û��ˮ��û������B.�������õ���ʳϰ�ߣ�����߲ˡ�ˮ���ȼ���ʳ��
C.����ˮԽ����Խ��D.��ζ����Ӫ�������Ǽӵ�Խ��Խ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ���仯�����ڹ�ҵ��������������;�dz��㷺���ش��������⣺
��1����̬��ͭ���Ӽ۵����Ų�ʽΪ____________����һ������I(Cu)________I (Zn)�������������ԭ��__________________________________________��
��2��Cu(CH3CN)42+���İ���ͭ���ӻ��ȶ�����������Cu����λ����_______��������̼ԭ�ӵ��ӻ�������_________________,1molCH3CN�ЦҼ��ĸ���Ϊ_____________��
��3��CuCl���л��ϳ��г���������CuCl�ۻ��������磬�Ʋ�CuCl�����л�ѧ������Ϊ_________��CuCl��ǿ�Ȼ�ֽ�����ͭ��ͭ����Ķѻ���ʽΪ__________�������ֱ�ʾ����
��4��������Ľṹ����ʯ�Ľṹ���ƣ���������ϵ����ͼ�������ʯ�����е�Cԭ��ȫ���û���Oԭ�ӣ�Oԭ�������������ĸ�Oԭ��������Hԭ�Ӳ�������������Oԭ��֮�䣬�����γ�һ�����ۼ���һ���������Ϊ���еĹ��ۼ��������0��ʱ������������ļ�����A��H��B��Ϊ_______________pm����ʽ�����㣩����0��ʱ���ܶ�Ϊ0.9g�Bcm-3��![]()
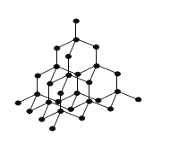
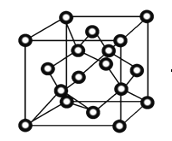
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȡ1�� 2-�������飬����ת����������õ���
A. CH3CH2Br![]() CH3CH2OH
CH3CH2OH![]() CH2=CH2
CH2=CH2![]() CH2BrCH2Br
CH2BrCH2Br
B. CH3CH2Br![]() CH2BrCH2Br
CH2BrCH2Br
C. CH3CH2Br![]() CH2=CH2
CH2=CH2![]() CH2BrCH3
CH2BrCH3![]() CH2BrCH2Br
CH2BrCH2Br
D. CH3CH2Br![]() CH2=CH2
CH2=CH2![]() CH2BrCH2Br
CH2BrCH2Br
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com