
��1����֪��N2��g����O2��g��===2NO��g������H��180��5 kJ��mol��1
4NH3��g����5O2��g��===4NO��g����6H2O��g������H����905 kJ��mol��1
2H2��g����O2��g��===2H2O��g������H����483��6 kJ��mol��1
��N2��g����3H2��g��![]() 2NH3��g���Ħ�H��______________��
2NH3��g���Ħ�H��______________��
(2) ���������������Ƚ������ⷽ������������������������������Ӧϵͳͬʱͨ����顢������ˮ��������������Ҫ��ѧ��Ӧ�У�
��Ӧ���� | ��ѧ����ʽ | �ʱ��H(kJ/mol) | ���Ea(kJ/mol) |
�������� | CH4(g)��2O2(g)��CO2(g)��2H2O(g) | ��802.6 | 125.6 |
CH4(g)��O2(g)��CO2(g)��2H2(g) | ��322.0 | 172.5 | |
�������� | CH4(g)��H2O(g)��CO(g)��3H2(g) | 206.2 | 240.1 |
CH4(g)��2H2O(g)��CO2(g)��4H2(g) | 165.0 | 243.9 |
�ش��������⣺
��ӦCO(g)��H2O(g)��CO2(g)��H2(g)����H=�������� kJ/mol
��3�� ��ͼ��ʾװ�ÿ������Ʊ�N2O5����N2O5�ڵ��ص�______������������������������ɣ���缫��ӦʽΪ______________________________��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺| t/s | 0 | 500 | 1000 |
| c��N2O5��/mol?L-1 | 5.00 | 3.52 | 2.48 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
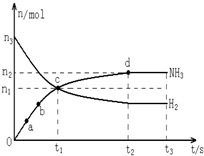 ��1913�깤ҵ�ϳɰ�Ͷ���������ϳɰ���ҵ���Ϸ�չ�����ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ�Ͽɽ���������������ش��������⣺
��1913�깤ҵ�ϳɰ�Ͷ���������ϳɰ���ҵ���Ϸ�չ�����ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ�Ͽɽ���������������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| b |
| 2 |
| 3c |
| 2 |
| b |
| 2 |
| 3c |
| 2 |
| 1 |
| 32 |
| 1 |
| 32 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| c��NO������10-4mol?L-1�� | 10.0 | 4.50 | 2.50 | 1.50 | 1.00 | 1.00 |
| c��NO������10-3mol?L-1�� | 3.60 | 3.05 | 2.85 | 2.75 | 2.70 | 2.70 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| O | 2- 3 |
| O | - 3 |
| H | + 4 |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com