
(2)20 mL 1 mol¡¤L-1µÄNaClºÍ40 mL 0.5 mol¡¤L-1 CaCl2»ìºÏºóµÄÈÜÒºÖУ¬Cl-µÄÎïÖʵÄÁ¿Å¨¶ÈÊÇ_________¡£
(3)ÔÚ±ê×¼×´¿öÏ£¬700 L NH3µÄÎïÖʵÄÁ¿Îª_________£¬È«²¿ÈܽâÔÚ1 LË®ÖУ¬ËùµÃÈÜÒºµÄÖÊÁ¿·ÖÊýΪ_________£¬Èç¹û¸Ã°±Ë®µÄÃܶÈΪ0.85 g¡¤cm-3£¬Ôò°±Ë®µÄÌå»ýΪ_________ L£¬ÎïÖʵÄÁ¿Å¨¶ÈΪ_________¡£
(4)ÒÑÖªÖÊÁ¿·ÖÊýΪ37%µÄŨÑÎËáµÄÃܶÈΪ1.19 g¡¤cm-3£¬¸ÃÑÎËáµÄÎïÖʵÄÁ¿Å¨¶ÈΪ_________¡£ÅäÖÆ200 mL 0.5 mol¡¤L-1ÑÎËáÈÜÒºÐèÒª¸ÃŨÑÎËá_________mL¡£
(5)³£ÎÂÏ£¬Ïà¶Ô·Ö×ÓÖÊÁ¿ÎªMµÄijÎÞË®ÑÎAµÄÈܽâ¶ÈΪS g£¬Ôò³£ÎÂʱ£¬¸ÃÑα¥ºÍÈÜÒºµÄÖÊÁ¿·ÖÊýΪ_________£¬Èç¹ûÒÑÖª¸Ã±¥ºÍÈÜÒºµÄÃܶÈΪ¦Ñ g¡¤cm-3£¬Ôò¸ÃÈÜÒºµÄÎïÖʵÄÁ¿Å¨¶ÈΪ_________¡£
 ÌìÌìÏòÉÏÒ»±¾ºÃ¾íϵÁдð°¸
ÌìÌìÏòÉÏÒ»±¾ºÃ¾íϵÁдð°¸ СѧÉú10·ÖÖÓÓ¦ÓÃÌâϵÁдð°¸
СѧÉú10·ÖÖÓÓ¦ÓÃÌâϵÁдð°¸
| Ä꼶 | ¸ßÖÐ¿Î³Ì | Ä꼶 | ³õÖÐ¿Î³Ì |
| ¸ßÒ» | ¸ßÒ»Ãâ·Ñ¿Î³ÌÍƼö£¡ | ³õÒ» | ³õÒ»Ãâ·Ñ¿Î³ÌÍƼö£¡ |
| ¸ß¶þ | ¸ß¶þÃâ·Ñ¿Î³ÌÍƼö£¡ | ³õ¶þ | ³õ¶þÃâ·Ñ¿Î³ÌÍƼö£¡ |
| ¸ßÈý | ¸ßÈýÃâ·Ñ¿Î³ÌÍƼö£¡ | ³õÈý | ³õÈýÃâ·Ñ¿Î³ÌÍƼö£¡ |
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º ÌâÐÍ£ºÔĶÁÀí½â
| c(CH3OH) |
| c(CO)¡Ác2(H2) |
| c(CH3OH) |
| c(CO)¡Ác2(H2) |
| b-a-4c |
| 2 |
| b-a-4c |
| 2 |
²é¿´´ð°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º ÌâÐÍ£ºÔĶÁÀí½â
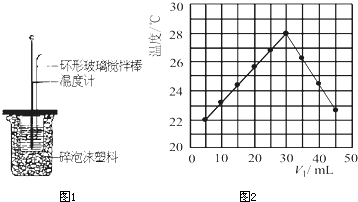
| ÎÂ¶È | ÆðʼζÈt1/¡æ | ÖÕÖ¹ÎÂ¶È t1/¡æ |
ÎÂ¶È²î £¨t2-t1£©t1/¡æ | ||
| ʵÑé´ÎÊý | H2SO4 | NaOH | ƽ¾ùÖµ | ||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | 4.0 4.0 |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
²é¿´´ð°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º ÌâÐÍ£ºÔĶÁÀí½â

| ´ß»¯¼Á |
| ¸ßθßѹ |
| ´ß»¯¼Á |
| ¸ßθßѹ |
| ÔÁÏ | ÌìÈ»Æø | ÖØÓÍ | ú |
| Ïà¶ÔͶ×Ê·ÑÓà | 1.0 | 1.5 | 2.0 |
| ÄÜÁ¿ÏûºÄ/J?t-1 | 28109 | 38109 | 48109 |
| ||
| ´ß»¯¼Á |
| ||
| ´ß»¯¼Á |
| ||
| ||
²é¿´´ð°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£ºÄþÏÄÒø´¨¶þÖÐ2011½ì¸ßÈýµÚÈý´ÎÔ¿¼»¯Ñ§ÊÔÌâ ÌâÐÍ£º022
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
²é¿´´ð°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º ÌâÐÍ£º
A.10 mL 0.1 mol¡¤L-1 NH4ClÈÜÒºÓë5 mL 0.2 mol¡¤L-1 NaOHÈÜÒº»ìºÏ£¬c(Na+)£¾c(Cl-)£¾c(OH-)£¾c(H+)
B.10 mL 0.1 mol¡¤L-1°±Ë®Óë10 mL 0.1 mol¡¤L-1ÑÎËá»ìºÏ£¬c(Cl-)£¾c(NH![]() )£¾c(OH-)£¾c(H+)
)£¾c(OH-)£¾c(H+)
C.10 mL 0.1 mol¡¤L-1 CH3COOHÈÜÒºÓë5 mL 0.2 moL¡¤L-1 NaOHÈÜÒº»ìºÏ£¬c(Na+)£¾c(CH3COO-)£¾c(OH-)£¾c(H+)
D.10 mL 0.5 mol¡¤L-1 CH3COONaÈÜÒºÓë6 mL 1 mol¡¤L-1ÑÎËá»ìºÏ£¬c(Cl-)£¾c(Na+)£¾c(OH-)£¾c(H+)
²é¿´´ð°¸ºÍ½âÎö>>
¹ú¼ÊѧУÓÅÑ¡ - Á·Ï°²áÁбí - ÊÔÌâÁбí
ºþ±±Ê¡»¥ÁªÍøÎ¥·¨ºÍ²»Á¼ÐÅÏ¢¾Ù±¨Æ½Ì¨ | ÍøÉÏÓк¦ÐÅÏ¢¾Ù±¨×¨Çø | µçÐÅթƾٱ¨×¨Çø | ÉæÀúÊ·ÐéÎÞÖ÷ÒåÓк¦ÐÅÏ¢¾Ù±¨×¨Çø | ÉæÆóÇÖȨ¾Ù±¨×¨Çø
Î¥·¨ºÍ²»Á¼ÐÅÏ¢¾Ù±¨µç»°£º027-86699610 ¾Ù±¨ÓÊÏ䣺58377363@163.com