
���п�ͼ�е���ĸ�ֱ����һ�ֳ��������ʻ�����Һ���֮���ת����ϵ��ͼ��ʾ�����ֲ��P��Ӧ��������ȥ������֪A��BΪ��̬���ʣ�F�ǵؿ��к������Ľ���Ԫ�صĵ��ʣ�E��H��IΪ�����EΪ��ɫ���壬IΪ����ɫ���壬MΪ���ɫ������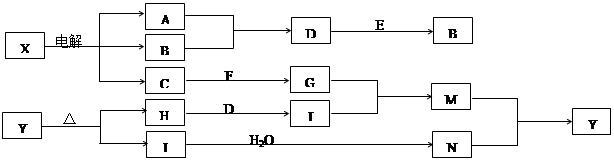
��1��B������Ԫ��λ�����ڱ��� �塣 ��2��A��B��ȼ�յ������� ��
��3��D + E �� B�ķ�Ӧ�У��������뱻��ԭ�����ʵ���֮���� ��
��4��G + J �� M�����ӷ���ʽ�� ����5��Y���ȷֽ�Ļ�ѧ����ʽ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ������Чʵ��ѧ������ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��8�֣�
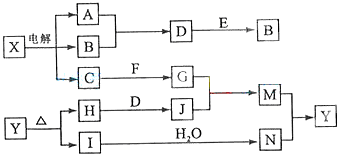
���п�ͼ�е���ĸ�ֱ����һ�ֳ��������ʻ�����Һ���֮���ת����ϵ����ͼ��ʾ�����ֲ��P��Ӧ��������ȥ������֪A��BΪ��̬���ʣ�F�ǵؿ��к������Ľ���Ԫ�صĵ��ʣ�E��H��IΪ�����EΪ��ɫ���壬IΪ����ɫ���壻MΪ���ɫ������
��ش��������⣺
��1��B������Ԫ��λ�����ڱ��е� ���ڣ� �塣
��2��A��B��ȼ�յ������� ��
��3��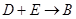 �ķ�Ӧ�У��������뱻��ԭ�����ʵ����ʵ������� ��
�ķ�Ӧ�У��������뱻��ԭ�����ʵ����ʵ������� ��
��4��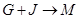 �����ӷ���ʽ�� ��
�����ӷ���ʽ�� ��
��5��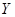 ���ȷֽ�Ļ�ѧ����ʽ�� ��
���ȷֽ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�������и���������¿���ѧ�Ծ����������� ���ͣ������
��14�֣����п�ͼ�е���ĸ�ֱ����һ�ֳ��������ʻ�����Һ���֮���ת����ϵ����ͼ��ʾ�����ֲ��P��Ӧ��������ȥ������֪A��BΪ��̬���ʣ�F�ǵؿ��к������Ľ���Ԫ�صĵ��ʣ�E��H��IΪ�����EΪ��ɫ���壬IΪ����ɫ���壻MΪ���ɫ������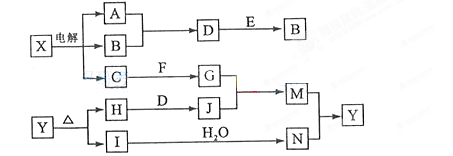
��ش��������⣺
��1��B������Ԫ��λ�����ڱ��� ���� ��
��д��C�ĵ���ʽ ��
��2��A��B��ȼ�յ������� ��
��3��D+E��B�ķ�Ӧ�У�n(������������)��n������ԭ�����ʣ�= ��
��4��G+J��M�����ӷ���ʽ�� ��
д��C+F��G�����ӷ���ʽ�� ��
��5�� ���ȷֽ�Ļ�ѧ����ʽ�� ��
���ȷֽ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������и����ڶ����¿���ѧ�Ծ��������棩 ���ͣ��ƶ���
���п�ͼ�е���ĸ�ֱ����һ�ֳ��������ʻ�����Һ���֮���ת����ϵ��ͼ��ʾ�����ֲ��P��Ӧ��������ȥ������֪A��BΪ��̬���ʣ�F�ǵؿ��к������Ľ���Ԫ�صĵ��ʣ�E��H��IΪ�����EΪ��ɫ���壬IΪ����ɫ���壬MΪ���ɫ������
��ش��������⣺
��1��B������Ԫ��λ�����ڱ��е� ���� �塣
��2�����X��Һ�����ӷ���ʽ�� ��
��3��D + E �� B�ķ�Ӧ�У��������뱻��ԭ�����ʵ����ʵ���֮���� ��
��4��G + J �� M�����ӷ���ʽ�� ��
��5��Y���ȷֽ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com