
Ρ≥Μ·―ß―ßœΑ–ΓΉι…ηΦΤœ¬ΆΦ Β―ιΉΑ÷Ο(Φ–≥÷ΉΑ÷Ο¬‘»Ξ)÷Τ±ΗCl2Θ§≤ΔΧΫΨΩ¬»ΤχΒΡœύΙΊ–‘÷ ΓΘ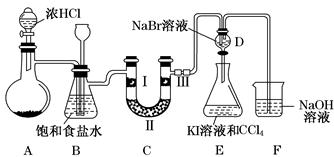
(1)»τAΉΑ÷Ο÷–ΙΧΧε“©ΤΖΈΣKClO3Θ§‘ρΖ¥”Π÷–ΟΩ…ζ≥…1 mol Cl2 ±ΉΣ“ΤΒγΉ”ΒΡΈο÷ ΒΡΝΩΈΣ________molΓΘ
(2)ΉΑ÷ΟBΦ»Ρή≥ΐ»Ξ¬»Τχ÷–ΒΡ¬»Μ·«βΘ§ΜΙΡήΦλ≤β Β―ιΙΐ≥Χ÷–ΉΑ÷ΟC «ΖώΖΔ…ζΕ¬»ϊΓΘ»τC÷–ΖΔ…ζΕ¬»ϊΘ§‘ρB÷–ΫΪΙέ≤λΒΫΒΡœ÷œσ «_______________________________________ΓΘ
(3)ΉΑ÷ΟCΒΡΉς”Ο «―ι÷Λ¬»Τχ «ΖώΨΏ”–Τ·ΑΉ–‘Θ§Δώ¥Π « Σ»σΒΡ”–…Ϊ≤ΦΧθΘ§‘ρΔρΓΔΔσ¥Π”ΠΦ”»κΒΡΈο÷ Ζ÷±π «________ΓΔ________ΓΘ
(4)…ηΦΤΉΑ÷ΟDΓΔEΒΡΡΩΒΡ «±»Ϋœ¬»ΓΔδεΓΔΒβΒΡΖ«Ϋπ τ–‘ΓΘ«κΦρ ωΡήΥΒΟς¬»ΓΔδεΓΔΒβΖ«Ϋπ τ–‘«Ω»θΒΡ Β―ι≤ΌΉςΦΑœ÷œσΘΚ_____________________________________________ΓΘ
(5)«κ”ΟΜ·―ßΖΫ≥Χ ΫΥΒΟςΉΑ÷ΟFΒΡΉς”ΟΘΚ_______________________________________ΓΘ
(6)ΦΉΆ§―ßΧα≥ωΘ§ΉΑ÷ΟF÷–ΒΡ ‘ΦΝΩ…ΗΡ”ΟΉψΝΩΒΡNa2SO3»ή“ΚΘ§““Ά§―ß»œ’φΥΦΩΦΚσ»œΈΣ¥ΥΖ®≤ΜΩ…––ΓΘ«κ”ΟάκΉ”ΖΫ≥Χ ΫΫβ Ά““»œΈΣ≤ΜΩ…––ΒΡ‘≠“ρΘΚ_______________________ΓΘ
(1)5/3ΓΓ(2)B÷–≥ΛΨ±¬©ΕΖ÷–“ΚΟφ…œ…ΐΘ§–Έ≥…“Κ÷υΓΓ(3)ΈόΥ°¬»Μ·ΗΤ(ΜρΙηΫΚΓΔP2O5)ΓΓΗ…‘οΒΡ”–…Ϊ≤ΦΧθ
(4)¥ρΩΣA÷–Ζ÷“Κ¬©ΕΖΒΡΜν»ϊΘ§“ΜΕΈ ±ΦδΚσΘ§D÷–ΒΡΈό…Ϊ»ή“Κ±δΈΣ≥»…Ϊ(≥»Κλ…ΪΜρΜΤ…Ϊ)Θ§ΥΒΟς¬»ΒΡΖ«Ϋπ τ–‘«Ω”ΎδεΘΜ¥ρΩΣD÷–Μν»ϊΘ§ΫΪD÷–…ΌΝΩ»ή“ΚΖ≈»κΉΑ÷ΟE÷–Θ§’ώΒ¥Θ§œ¬≤ψΈΣΉœΚλ…ΪΘ§ΥΒΟςδεΒΡΖ«Ϋπ τ–‘«Ω”ΎΒβ
(5)Cl2ΘΪ2NaOH=NaClΘΪNaClOΘΪH2O
(6)SO32ΓΣΘΪCl2ΘΪH2O=SO42ΓΣΘΪ2ClΘ≠ΘΪ2HΘΪΓΔSO32ΓΣΘΪ2HΘΪ=SO2ΓϋΘΪH2O
ΫβΈω


 ΩΎΥψΧβΩ®±±Ψ©ΗΨ≈°ΕυΆ·≥ωΑφ…γœΒΝ–¥πΑΗ
ΩΎΥψΧβΩ®±±Ψ©ΗΨ≈°ΕυΆ·≥ωΑφ…γœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ Β―ιΧβ
ΒΞΨßΙη «–≈œΔ≤ζ“Β÷–÷Ί“ΣΒΡΜυ¥Γ≤ΡΝœΓΘΆ®≥Θ”ΟΧΦ‘ΎΗΏΈ¬œ¬ΜΙ‘≠Εΰ―θΜ·Ιη÷ΤΒΟ¥÷Ιη(Κ§ΧζΓΔ¬ΝΓΔ≈πΓΔΝΉΒ»‘”÷ )Θ§¥÷Ιη”ꬻΤχΖ¥”Π…ζ≥…ΥΡ¬»Μ·Ιη(Ζ¥”ΠΈ¬Ε»450ΓΪ500 Γφ)Θ§ΥΡ¬»Μ·ΙηΨ≠Χα¥ΩΚσ”Ο«βΤχΜΙ‘≠Ω…ΒΟ
ΗΏ¥ΩΙηΓΘ“‘œ¬ « Β―ι “÷Τ±ΗΥΡ¬»Μ·ΙηΒΡΉΑ÷Ο Ψ“βΆΦΓΘ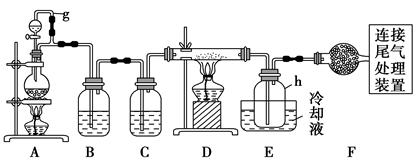
œύΙΊ–≈œΔ»γœ¬ΘΚ
aΘ°ΥΡ¬»Μ·Ιη”ωΥ°ΦΪ“ΉΥ°ΫβΘΜ
bΘ°≈πΓΔ¬ΝΓΔΧζΓΔΝΉ‘ΎΗΏΈ¬œ¬ΨυΡޔꬻΤχ÷±Ϋ”Ζ¥”Π…ζ≥…œύ”ΠΒΡ¬»Μ·ΈοΘΜ
cΘ°”–ΙΊΈο÷ ΒΡΈοάμ≥Θ ΐΦϊœ¬±μΘΚ
| Έο÷ | SiCl4 | BCl3 | AlCl3 | FeCl3 | PCl5 |
| Ζ–Βψ/Γφ | 57.7 | 12.8 | Θ≠ | 315 | Θ≠ |
| »έΒψ/Γφ | Θ≠70.0 | Θ≠107.2 | Θ≠ | Θ≠ | Θ≠ |
| …ΐΜΣΈ¬Ε»/Γφ | Θ≠ | Θ≠ | 180 | 300 | 162 |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ Β―ιΧβ
ΗυΨίœ¬ΆΦΉΑ÷ΟΫχ–– Β―ιΘ§“―÷ΣΘΚNa2O2”κH2OΚΆCO2ΕΦΡήΖ¥”Π≤Δ…ζ≥…O2,ΒΪ”κNH3≤ΜΖ¥”Π
ΜΊ¥πœ¬Ν–Έ ΧβΘΚΓΘ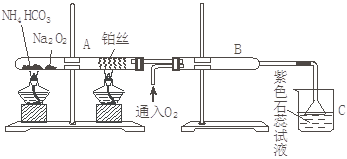
Θ®1Θ©‘Ύ ή»»ΒΡ ‘ΙήA÷–NH4HCO3ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ ΓΘ
Θ®2Θ©±ΜΦ”»»ΒΡ≤§ΥΩ¥ΠΖΔ…ζΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ___________________________________ΓΘ
Θ®3Θ©B÷–≥ωœ÷ΒΡœ÷œσΈΣΘΚ___________________________________________________ΓΘ
Θ®4Θ©…’±≠C÷–ΖΔ…ζΒΡœ÷œσΈΣ________________________________________________ΓΘ
Θ®5Θ©ΒΙ÷Ο¬©ΕΖΒΡΉς”Ο ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ Β―ιΧβ
Α±Τχ‘ΎΙΛ≈©“Β…ζ≤ζ÷–”–÷Ί“ΣΒΡ”ΟΆΨΓΘΡ≥–ΘΦΉΓΔ““ΝΫΗωΜ·―ß–ΓΉιΖ÷±πΕ‘Α±ΒΡœύΙΊ Β―ιΫχ––ΝΥ―–ΨΩΓΘ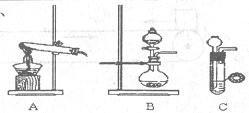
Θ®1Θ©ΦΉΓΔ““ΝΫ–ΓΉι―Γ‘ώΝΥ≤ΜΆ§ΖΫΖ®÷Τ»ΓΑ±ΤχΘ§
«κΫΪ Β―ιΉΑ÷ΟΒΡΉ÷ΡΗ±ύΚ≈ΚΆ÷Τ±Η‘≠άμΧν–¥‘Ύœ¬±μΩ’Ηώ÷–ΓΘ
| | Β―ιΉΑ÷Ο | Β―ι“©ΤΖ | ÷Τ±Η‘≠άμ |
| ΦΉ–ΓΉι | A | «β―θΜ·ΗΤΓΔ¬»Μ·οß | Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ΔΌ |
| ““–ΓΉι | ΔΎ | ≈®Α±Υ°ΓΔ«β―θΜ·ΡΤ | ”ΟΜ·―ßΤΫΚβ‘≠άμΖ÷Έω«β―θΜ·ΡΤΒΡΉς”ΟΘΚ Δέ |

 3Cu+N2+3H2OΘ©Θ§ΕχΒΣΤχΗζΟΨ‘ΎΗΏΈ¬œ¬Ζ¥”ΠΩ…ΒΟΒΫΒΣΜ·ΟΨΘ§ΒΪΒΣΜ·ΟΨ”ωΥ°ΝΔΦ¥Ζ¥”Π…ζ≥…MgΘ®OHΘ©2ΚΆNH3ΓΘ““ΉιΧα≥ωΝΥ»γœ¬÷Τ±ΗΒΣΜ·ΟΨΒΡ Β―ιΖΫΑΗ Ψ“βΩρΆΦΘ® Β―ι«ΑœΒΆ≥ΡΎΩ’Τχ“―≈≈≥ΐΘΜΆΦ÷–ΦΐΆΖ±μ ΨΤχΧεΒΡΝςœρΘ©ΓΘΡψ»œΈΣ¥ΥΖΫΑΗ «Ζώ’ΐ»ΖΘ§≤ΔΥΒΟςάμ”… ΓΘ
3Cu+N2+3H2OΘ©Θ§ΕχΒΣΤχΗζΟΨ‘ΎΗΏΈ¬œ¬Ζ¥”ΠΩ…ΒΟΒΫΒΣΜ·ΟΨΘ§ΒΪΒΣΜ·ΟΨ”ωΥ°ΝΔΦ¥Ζ¥”Π…ζ≥…MgΘ®OHΘ©2ΚΆNH3ΓΘ““ΉιΧα≥ωΝΥ»γœ¬÷Τ±ΗΒΣΜ·ΟΨΒΡ Β―ιΖΫΑΗ Ψ“βΩρΆΦΘ® Β―ι«ΑœΒΆ≥ΡΎΩ’Τχ“―≈≈≥ΐΘΜΆΦ÷–ΦΐΆΖ±μ ΨΤχΧεΒΡΝςœρΘ©ΓΘΡψ»œΈΣ¥ΥΖΫΑΗ «Ζώ’ΐ»ΖΘ§≤ΔΥΒΟςάμ”… ΓΘ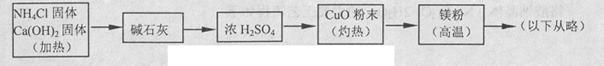
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ Β―ιΧβ
ΈΣΝΥΧΫΨΩAgNO3ΒΡ―θΜ·–‘ΚΆ»»Έ»Ε®–‘Θ§Ρ≥Μ·―ß–Υ»Λ–ΓΉι…ηΦΤΝΥ»γœ¬ Β―ιΓΘ
Δώ.AgNO3ΒΡ―θΜ·–‘
ΫΪΙβΝΝΒΡΧζΥΩ…λ»κAgNO3»ή“Κ÷–Θ§“ΜΕΈ ±ΦδΚσΫΪΧζΥΩ»Γ≥ωΓΘΈΣΦλ―ι»ή“Κ÷–FeΒΡ―θΜ·≤ζΈοΘ§ΫΪ»ή“Κ÷–ΒΡAgΘΪ≥ΐΨΓΚσΘ§Ϋχ––ΝΥ»γœ¬ Β―ιΘ§Ω…―Γ”Ο ‘ΦΝΘΚKSCN»ή“ΚΓΔK3[Fe(CN)6]»ή“ΚΓΔ¬»Υ°ΓΘ
(1)«κΆξ≥…œ¬±μΘΚ
| ≤ΌΉς | œ÷œσ | Ϋα¬έ |
| »Γ…ΌΝΩ≥ΐΨΓAgΘΪΚσΒΡ»ή“Κ”Ύ ‘Ιή÷–Θ§Φ”»κKSCN»ή“ΚΘ§’ώΒ¥ | | ¥φ‘ΎFe3ΘΪ |
| »Γ…ΌΝΩ≥ΐΨΓAgΘΪΚσΒΡ»ή“Κ”Ύ ‘Ιή÷–Θ§Φ”»κ________Θ§’ώΒ¥ | | ¥φ‘ΎFe2ΘΪ |

| Β―ι±ύΚ≈ | ≤ΌΉς | œ÷œσ |
| a | Φ”»κΉψΝΩΑ±Υ°Θ§’ώΒ¥ | ΚΎ…ΪΙΧΧε≤Μ»ήΫβ |
| b | Φ”»κΉψΝΩœΓœθΥαΘ§’ώΒ¥ | ΚΎ…ΪΙΧΧε»ήΫβΘ§≤Δ”–ΤχΧε≤ζ…ζ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ Β―ιΧβ
Ρ≥ΩΈΆβΜνΕ·–ΓΉι”ϊάϊ”ΟCuO”κNH3Ζ¥”ΠΘ§―–ΨΩNH3ΒΡΡ≥÷÷–‘÷ ≤Δ≤βΕ®ΤδΉι≥…Θ§…ηΦΤΝΥ»γœ¬ Β―ιΉΑ÷Ο(Φ–≥÷ΉΑ÷ΟΈ¥Μ≠≥ω)Ϋχ–– Β―ιΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ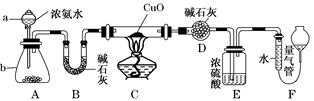
Θ®1Θ©“«ΤςaΒΡΟϊ≥ΤΈΣ________ΘΜ“«Τςb÷–Ω…―Γ‘ώΒΡ ‘ΦΝΈΣ________ΓΘ
Θ®2Θ© Β―ι “÷–Θ§άϊ”ΟΉΑ÷ΟAΜΙΩ…÷Τ»ΓΒΡΈό…ΪΤχΧε «________(ΧνΉ÷ΡΗ)
| AΘ°Cl2 | BΘ°O2 | CΘ°CO2 | DΘ°NO2 |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ Β―ιΧβ
ΈΣ―–ΨΩΆ≠”κ≈®ΝρΥαΒΡΖ¥”ΠΘ§Ρ≥Μ·―ß–Υ»Λ–ΓΉιΫχ––»γœ¬ Β―ιΓΘ
Β―ιΔώΓΓΖ¥”Π≤ζΈοΒΡΕ®–‘ΧΫΨΩ
Β―ιΉΑ÷Ο»γΆΦΥυ ΨΓΘΘ®ΙΧΕ®ΉΑ÷Ο“―¬‘»ΞΘ©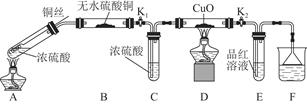
Θ®1Θ©A÷–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ΓΘ
Θ®2Θ©F…’±≠÷–ΒΡ»ή“ΚΆ®≥Θ « ΓΘ
Θ®3Θ© Β―ιΙΐ≥Χ÷–Θ§Ρή÷ΛΟς≈®ΝρΥα÷–Νρ‘ΣΥΊΒΡ―θΜ·–‘«Ω”Ύ«β‘ΣΥΊΒΡœ÷œσ « ΓΘ
Θ®4Θ© Β―ιΫα χΚσΘ§÷ΛΟςAΉΑ÷Ο ‘Ιή÷–Ζ¥”ΠΥυΒΟ≤ζΈο «ΖώΚ§”–Ά≠άκΉ”ΒΡ≤ΌΉςΖΫΖ® « ΓΘ
Θ®5Θ©ΈΣΥΒΟς≈®ΝρΥα÷–ΒΡΥ° «Ζώ”ΑœλBΉΑ÷Οœ÷œσΒΡ≈–ΕœΘ§ΜΙ–κΫχ––“Μ¥Έ Β―ιΓΘ Β―ιΖΫΑΗΈΣ ΓΘ
Β―ιΔρΓΓΖ¥”Π≤ζΈοΒΡΕ®ΝΩΧΫΨΩ
Θ®6Θ©‘ΎΆ≠”κ≈®ΝρΥαΖ¥”ΠΒΡΙΐ≥Χ÷–Θ§ΖΔœ÷”–ΚΎ…ΪΈο÷ ≥ωœ÷Θ§Ψ≠≤ι‘ΡΈΡœΉΜώΒΟœ¬Ν–Ή ΝœΓΘ
Ή Νœ1ΘΚ
| ΝρΥα/molΓΛLΘ≠1 | ΚΎ…ΪΈο÷ ≥ωœ÷ΒΡΈ¬Ε»/Γφ | ΚΎ…ΪΈο÷ œϊ ßΒΡΈ¬Ε»/Γφ |
| 15 | ‘Φ150 | ‘Φ236 |
| 16 | ‘Φ140 | ‘Φ250 |
| 18 | ‘Φ120 | ≤Μœϊ ß |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ Β―ιΧβ
Ρ≥Μ·―ß–Υ»Λ–ΓΉιΈΣ―ι÷ΛNO2ΒΡ―θΜ·–‘ΚΆNOΒΡΜΙ‘≠–‘Θ§…ηΦΤΝΥ»γœ¬ΉΑ÷Ο÷Τ»ΓNO2ΚΆNOΘ§≤Δ―ι÷ΛΤδ–‘÷ ΘΚ
(1)–¥≥ωΦΉ÷–Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ_________________________________Θ§
““÷–ΒΡœ÷œσ «_________________________________________________Θ§
Ω…÷ΛΟςNO2ΒΡ―θΜ·–‘ΘΜ‘Ύ±ϊ÷–ΙΡ»κΩ’ΤχΚσΒΡœ÷œσ «_______________Θ§Ω…÷ΛΟςNOΒΡΜΙ‘≠–‘ΓΘ
(2) Β―ι«Α±ϊ÷–≥δ¬ζΥ°ΒΡΉς”Ο «___________________________________
(”ΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΚΆΦρ“ΣΈΡΉ÷ΜΊ¥π)ΓΘ
(3)–ΓΜΣΕ‘…œ ω Β―ι…ηΦΤΧα≥ωΝΥ÷ “…Θ§Υϊ»œΈΣ““÷–ΒΡœ÷œσ≤ΜΉψ“‘÷ΛΟςNO2ΒΡ―θΜ·–‘Θ§ΥϊΒΡάμ”… «___________________________________________ΓΘ
Ρψ»œΈΣ‘θ―υ≤≈ΡήΉΦ»Ζ÷ΛΟςNO2ΒΡ―θΜ·–‘ΘΩ____________________________________(Φρ“ΣΜΊ¥π≥ω‘≠άμΚΆœ÷œσΦ¥Ω…)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ Β―ιΧβ
Ρ≥Μ·―ßΩΈΆβΜνΕ·–ΓΉιΆ®Ιΐ Β―ι―–ΨΩNO2ΒΡ–‘÷ Θ°
“―÷ΣΘΚ2NO2+2NaOH®TNaNO3+NaNO2+H2O
άϊ”ΟΆΦ1Υυ ΨΉΑ÷ΟΧΫΨΩNO2ΡήΖώ±ΜNH3ΜΙ‘≠Θ®K1ΓΔK2ΈΣ÷ΙΥ°Φ–Θ§Φ–≥÷ΙΧΕ®ΉΑ÷Ο¬‘»ΞΘ©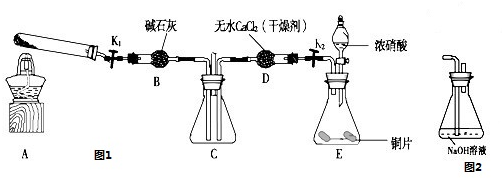
Θ®1Θ©EΉΑ÷Ο÷–÷Τ»ΓNO2Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «
Θ®2Θ©ΗΟ Β―ι÷–÷Τ»ΓΑ±Τχ ±»τ÷Μ”Ο“Μ÷÷ ‘ΦΝΘ§¥”œ¬Ν–Έο÷ ÷–―ûà Ȯ Θ©
aΘ°NH4HCO3 bΘ°NH4Cl cΘ°≈®Α±Υ°
Θ®3Θ©»τNO2ΡήΙΜ±ΜNH3ΜΙ‘≠Θ§‘ΛΤΎΙέ≤λΒΫCΉΑ÷Ο÷–ΒΡœ÷œσ «
Θ®4Θ©¥Υ Β―ιΉΑ÷Ο¥φ‘Ύ“ΜΗωΟςœ‘ΒΡ»±œί «
Θ®5Θ©ΧΫΨΩNO2ΡήΖώ”κNa2O2ΖΔ…ζ―θΜ·ΜΙ‘≠Ζ¥”ΠΘ°ΈΣΝΥ―ι÷ΛNO2Ρή±ΜNa2O2―θΜ·Θ§ΗΟ–ΓΉιΆ§―ß―Γ”ΟBΓΔDΓΔEΉΑ÷ΟΘ§ΫΪB÷–ΒΡ“©ΤΖΗϋΜΜΈΣNa2O2Θ§Νμ―ΓFΉΑ÷ΟΘ®»γΆΦ2Υυ ΨΘ©Θ§÷Ί–¬ΉιΉΑΘ§Ϋχ–– Β―ιΘ°ΉΑ÷ΟΒΡΚœάμΝ§Ϋ”Υ≥–ρ «
Θ®6Θ© Β―ιΙΐ≥Χ÷–Θ§BΉΑ÷Ο÷–Β≠ΜΤ…ΪΖέΡ©÷π ΫΞ±δ≥…ΑΉ…ΪΘ°Ψ≠Φλ―ιΘ§ΗΟΑΉ…ΪΈο÷ ΈΣ¥ΩΨΜΈοΘ§«“ΈόΤδΥϊΈο÷ …ζ≥…Θ°ΆΤ≤βBΉΑ÷Ο÷–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com