
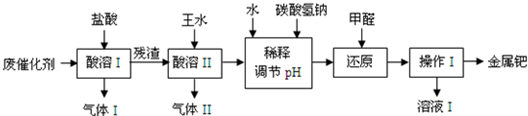
��16�֣���(Pd)�������벬���ơ���ҵ�ϴӷϴ�������Ҫ�ɷ����ٺͻ���̿����������������п���л����٣������������̣�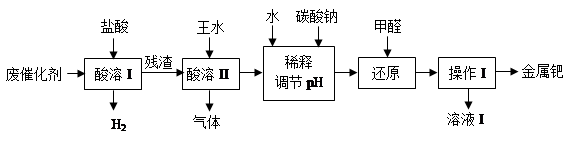
��ش��������⣺
��1������I��Ŀ����__________________________________��
��2�������ܢ�ʱ��������ˮ�ڼ���������������Ҫ��Ӧ�ǣ�
3Pd +12HCl + 2HNO3  3H2PdCl4 + 2NO��+ 4H2O
3H2PdCl4 + 2NO��+ 4H2O
д����������һ��Ҫ�ɷ���Ũ���ᷴӦ�Ļ�ѧ����ʽ��_________________��
�����ܢ�������¶Ȳ��˹��ߣ����˿���һ����Ӧ�����⣬��ԭ����ܻ���
_____________________________��
�������������ռ��������壬��д�����պ���Һ�к��е����ʵĻ�ѧʽ��NaOH��NaNO2��___________��___________��
��3��ʹ�ü�ȩ��ԭ�ٵĻ�����ʱ����Һ�뱣�ּ��ԣ��������ɼ�ȩ�Ķ�����ģ�ԭ����_______________________________________��
��4������I��������_______________����ҺI���ܺ��е��л�����Ϊ________________��
��5������������ڽ�������ǰ����Ƚ��ϴ�����700���½������գ�ͬʱ����ͨ�������
��Ŀ����______________________________________________________��
 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ�����߿�ģ�������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
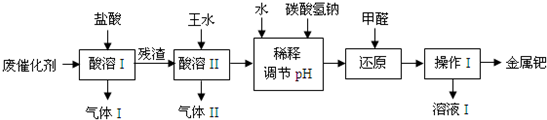
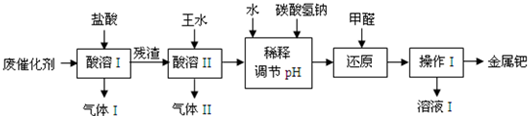
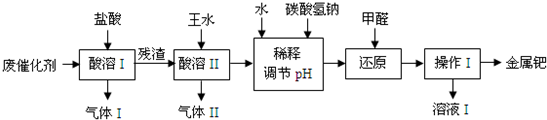
��16�֣���(Pd)�������벬���ơ���ҵ�ϴӷϴ�������Ҫ�ɷ����ٺͻ���̿����������������п���л����٣������������̣�

��ش��������⣺
��1������I��Ŀ����__________________________________��
��2�������ܢ�ʱ��������ˮ�ڼ���������������Ҫ��Ӧ�ǣ�
3Pd
+12HCl + 2HNO3  3H2PdCl4
+ 2NO��+ 4H2O
3H2PdCl4
+ 2NO��+ 4H2O
д����������һ��Ҫ�ɷ���Ũ���ᷴӦ�Ļ�ѧ����ʽ��_________________��
�����ܢ�������¶Ȳ��˹��ߣ����˿���һ����Ӧ�����⣬��ԭ����ܻ���
_____________________________��
�������������ռ��������壬��д�����պ���Һ�к��е����ʵĻ�ѧʽ��NaOH��NaNO2��___________��___________��
��3��ʹ�ü�ȩ��ԭ�ٵĻ�����ʱ����Һ�뱣�ּ��ԣ��������ɼ�ȩ�Ķ�����ģ�ԭ����_______________________________________��
��4������I��������_______________����ҺI���ܺ��е��л�����Ϊ________________��
��5������������ڽ�������ǰ����Ƚ��ϴ�����700���½������գ�ͬʱ����ͨ�������
��Ŀ����______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com