
| ʵ���� | ʵ��Ŀ�� | �¶� | c��NH4HCO3�� |
| I | Ϊ����ʵ�������� | 60�� | c1 |
| II | ̽��Ũ�ȶԷ�Ӧ�����ʵ�Ӱ�� | | c2 |
| III | ̽���¶ȶԷ�Ӧ�����ʵ�Ӱ�� | 80�� | |

 +H2O
+H2O NH3��H2KO+H+
NH3��H2KO+H+ +H2O
+H2O H2CO3+OH
H2CO3+OH
 ��HCO
��HCO ��ˮ��̶Ȼ�����ͬ
��ˮ��̶Ȼ�����ͬ NH3?H2O+H+��HCO3-+H2O
NH3?H2O+H+��HCO3-+H2O H2CO3+OH-��NH4+��HCO3-��ˮ��̶Ȼ�����ͬ��
H2CO3+OH-��NH4+��HCO3-��ˮ��̶Ȼ�����ͬ��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1��1 | B��2��1 | C��3��2 | D��1��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����켦�����������,��������Һ�з���������������Ǧ��Һ�б��� |
| B�����켦������ʳ���п��ܻᡰ���衱 |
| C������ʳ�����켦�����Բ��� |
| D�����켦�����ڷ��û��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
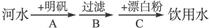
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʯ��ˮ��ͨ�����������̼ | B���Ȼ�����Һ�мӰ�ˮ |
| C��̼������Һ�м����� | D��BaCl2��Һ��ͨ�������̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com