
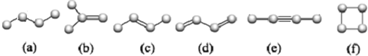
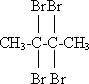
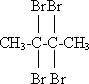
 ��
��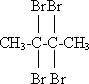 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| Ũ���� |
| Ũ���� |
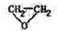
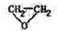
 ����
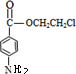
����

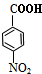 +HOCH2CH2Cl
+HOCH2CH2Cl| Ũ���� |
| �� |
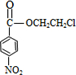 +H2O
+H2O +HOCH2CH2Cl
+HOCH2CH2Cl| Ũ���� |
| �� |
 +H2O
+H2O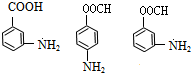

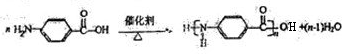
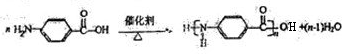
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ��������У������ѧ������������ѧ�Ծ��������棩 ���ͣ������
�йض�����Ԫ��A��B��C��D��E��F����Ϣ���£�
Ԫ�� �й���Ϣ
A ����������Ӧ��ˮ����ף���������̬�⻯��ң���Ӧ������
B �����������Ǵ�����������2��
C M������3������
D ������ԭ�Ӱ뾶��������Ԫ��
E �䵥���ǵ���ɫ����
F �������������۴�����Ϊ6
��ش��������⣺
��1��д��ʵ������ȡ�ҵĻ�ѧ����ʽ ��
��2������˵����ȷ���� (�����)��
�� ʵ���ҿ�����ͼ��ʾװ����ȡB�����������
�� ��C�������ɵIJ۳����������������Ũ��Һ
�� C��ͭ��ϡ������ɵ�ԭ��أ�C�缫����ԭ
�� D������������ȼ�պ�IJ�������ڷ����������������
�� �����������������У�������̼����ǿ��ƺ�����BO2���������ЧӦ������Ч;��֮һ
 �� DF�ĵ���ʽΪH��Cl��
�� DF�ĵ���ʽΪH��Cl��
��3����E�ij������������������ʹƷ����Һ��ɫ��ͨ����CuSO4��NaCl��ϵ�Ũ��Һ�У���Һ��ɫ��dz��������ɫ������ȡ�ó�������Ԫ������������������֪���к�Cl��35.7%��Cu��64.3%�������������������Ӧ�е������� ��
A��Ư�� B�������� C����ԭ��
��4�����û�ѧ����������֤��3���е��������Ҫд��ʵ�鷽�����Լ���Ԥ�ڿɹ۲쵽������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��16�֣��±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�
| �� |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| �� |
|
|
|
|
|
|
|
|
|
|
| �� | �� | �� | �� |
|
|
| �� |
|
|
|
|
|
|
|
|
|
|
|
|
| �� |
|
|
|
|
|
|
|
| �� |
|
|
|
| �� |
|
|
|
|
|
|
|
��1��д���ϱ���Ԫ�آ�ԭ�ӵ���Χ�����Ų�ʽ ��
��2����Ԫ�آ�����γɵ�ˮ����������廯�����У�Ԫ�آ۵��ӻ���ʽΪ��
��3����Ҫ��������и���
a����һ�����ܣ�Ԫ�آ� Ԫ�آݣ�ѡ���������������������)��
b����Ԫ�آ����γɵĵ��ʻ�Ϊ�ȵ�����ķ��ӡ����ӵĻ�ѧʽ �� ����дһ�֣���
c��Ԫ�آܵ���̬�⻯��X��ˮ��Һ�����ӹ�ҵ�У�������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ________________________________
d����X��������KOH��Һ����ԭ��أ����������Ԫ�آܵĵ��ʡ����为����ӦʽΪ_____________________________��
��4����Ԫ�آۺ͢��γɵ�Һ̬������Z���ǷǼ��Ե�ֱ���η��ӡ�0.2mol��Z��O2����ȫȼ�գ�����������̬�����298Kʱ�ų�����215kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ_________________________
��5���ڲⶨ������γɻ��������Է�������ʱ��ʵ���õ�ֵһ���������ֵ����Ҫԭ���ǣ� ��
��6��Ԫ�آ����γɵĵ��ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�� ��֪��ԭ�ӵİ뾶Ϊd pm�����ԭ������ΪM��NA���������ӵ���������ش�
�����и�ԭ�ӵ���λ��Ϊ ��һ�������а�����ԭ����ĿΪ ���þ�����ܶ�Ϊ g��cm��3������ĸ��ʾ�����ػ���
������������Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ�á�����Ԫ�������ڱ��е����ʿ��жϣ�����H������Be������C������N������O������F������Mg����S������Cr������Cu��
��1����Ϊȫ�������������ȶ��ģ����Ը��ݹ���ԭ����֪Cr����Χ�����Ų�ʽ3d54s1��
��2��������γɵ�ˮ�����������ϩ����ϩ�к���̼̼˫�������õ���sp2�ӻ���
�ǽ�����Խǿ����һ������Խ������N��O�������к���14�����ӣ����Ժ͵�����Ϊ�ȵ�����ķ�����CO��������C22-��N���⻯���ǰ�����N�Ļ��ϼ۴�����ͼ�̬����˫��ˮ�������ɵ�����ԭ����и���ʧȥ���ӣ��������ڸ����ĵ缫��ӦʽΪ2NH3��6e����6OH��===N2��6H2O��
��4��S��C�γɵķǼ��Ե�ֱ���η�����CS2�����Է�Ӧ���Ȼ�ѧ����ʽΪCS2(l)��3O2(g)===CO2(g)��2SO2(g) ��H����1075 kJ/mol
��5��F������õķǽ���Ԫ�أ�H��F�γɵ��⻯���к���������Ӷ����²�õ�ֵһ���������ֵ��
��6��ͭ�γɵ��������������ܶѻ�������λ����12���������еĽṹ�ص��֪һ�������а�����ԭ����ĿΪ![]()
![]() 8��1/8��6��1/2��4�����ݱ�ͼ��֪�þ����ı߳�Ϊ
8��1/8��6��1/2��4�����ݱ�ͼ��֪�þ����ı߳�Ϊ ���������ܶ�Ϊ
���������ܶ�Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��16�֣��±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�
|
�� |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
�� |
|
|
|
|
|
|
|
|
|
|
|
�� |
�� |
�� |
�� |
|
|
|
�� |
|
|
|
|
|
|
|
|
|
|
|
|
|
�� |
|
|
|
|
|
|
|
|
�� |
|
|
|
|
�� |
|
|
|
|
|
|
|
��1��д���ϱ���Ԫ�آ�ԭ�ӵ���Χ�����Ų�ʽ ��
��2����Ԫ�آ�����γɵ�ˮ����������廯�����У�Ԫ�آ۵��ӻ���ʽΪ��
��3����Ҫ��������и���
a����һ�����ܣ�Ԫ�آ� Ԫ�آݣ�ѡ���������������������)��
b����Ԫ�آ����γɵĵ��ʻ�Ϊ�ȵ�����ķ��ӡ����ӵĻ�ѧʽ �� ����дһ�֣���
c��Ԫ�آܵ���̬�⻯��X��ˮ��Һ�����ӹ�ҵ�У�������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ________________________________
d����X��������KOH��Һ����ԭ��أ����������Ԫ�آܵĵ��ʡ����为����ӦʽΪ_____________________________��
��4����Ԫ�آۺ͢��γɵ�Һ̬������Z���ǷǼ��Ե�ֱ���η��ӡ�0.2mol��Z��O2����ȫȼ�գ�����������̬�����298Kʱ�ų�����215kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ_________________________
��5���ڲⶨ������γɻ��������Է�������ʱ��ʵ���õ�ֵһ���������ֵ����Ҫԭ���ǣ� ��
��6��Ԫ�آ����γɵĵ��ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�� ��֪��ԭ�ӵİ뾶Ϊd pm�����ԭ������ΪM��NA���������ӵ���������ش�

�����и�ԭ�ӵ���λ��Ϊ ��һ�������а�����ԭ����ĿΪ ���þ�����ܶ�Ϊ g��cm��3������ĸ��ʾ�����ػ���
������������Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ�á�����Ԫ�������ڱ��е����ʿ��жϣ�����H������Be������C������N������O������F������Mg����S������Cr������Cu��
��1����Ϊȫ�������������ȶ��ģ����Ը��ݹ���ԭ����֪Cr����Χ�����Ų�ʽ3d54s1��
��2��������γɵ�ˮ�����������ϩ����ϩ�к���̼̼˫�������õ���sp2�ӻ���
�ǽ�����Խǿ����һ������Խ������N��O�������к���14�����ӣ����Ժ͵�����Ϊ�ȵ�����ķ�����CO��������C22-��N���⻯���ǰ�����N�Ļ��ϼ۴�����ͼ�̬����˫��ˮ�������ɵ�����ԭ����и���ʧȥ���ӣ��������ڸ����ĵ缫��ӦʽΪ2NH3��6e����6OH��===N2��6H2O��
��4��S��C�γɵķǼ��Ե�ֱ���η�����CS2�����Է�Ӧ���Ȼ�ѧ����ʽΪCS2(l)��3O2(g)===CO2(g)��2SO2(g) ��H����1075 kJ/mol
��5��F������õķǽ���Ԫ�أ�H��F�γɵ��⻯���к���������Ӷ����²�õ�ֵһ���������ֵ��
��6��ͭ�γɵ��������������ܶѻ�������λ����12���������еĽṹ�ص��֪һ�������а�����ԭ����ĿΪ
 8��1/8��6��1/2��4�����ݱ�ͼ��֪�þ����ı߳�Ϊ
8��1/8��6��1/2��4�����ݱ�ͼ��֪�þ����ı߳�Ϊ ���������ܶ�Ϊ
���������ܶ�Ϊ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com