
���� 25��ʱ��CH3COONH4�����ԣ�˵���������ƽ�ⳣ����һˮ�ϰ�����ƽ�ⳣ����ȣ�
��1����0.1mol/L��CH3COOH��Һ��0.1mol/L��NaOH��Һ�������ϣ���Ϻ���Һ������仯���Բ��ƣ�������ǡ����ȫ��Ӧ���ɴ����ƣ���û����Һ��pH=9��˵���õ�������ǿ�������Σ����������ˮ�����Һ�ʼ��ԣ�
��2�������Һ�д��������غ㣬���������غ��c��CH3COOH��=c��OH-��-c��H+����
��3��CH3COONH4��Һ�����ԣ�˵��CH3COO-ˮ��̶Ⱥ�NH4+ˮ��̶���ͬ��CO32-ˮ��̶ȴ���CH3COO-�����Ԣ���Һ�ʼ��ԣ�
����Һ�����ԣ�
�ۢ���NH4+ˮ��̶���ͬ��������Һ�������ԣ�������NH4+Ũ�ȴ�����pH�ۣ��ܣ�
��4��Ϊ��֤��Mg��OH��2����Һ���Ȼ����Һ�ķ�Ӧԭ�����ɼ���������Һ���飬��������Һ�����ԣ����ͬѧ�Ľ�����ȷ����������þ���ܽ⣬��������þ�ܽ⣬����ͬѧ�Ľ�����ȷ��
��5����ͬѧ����ѡ�Լ��μӵ�Mg��OH��2����Һ�У�Mg��OH��2�ܽ⣬˵��������þ��笠����ӷ�Ӧ��ʹ������þ�ܽ⣻
��6��ͨ�����Ϸ���֪��������þ������Σ�����������������Σ���������γ�ȥ������þ����������������ǿ���������þ������ǿ����Կ�����ǿ����Һ��ȥ����������
��� �⣺��1�������ʵ����Ĵ����NaOHǡ����ȫ��Ӧ���ɴ����ƣ���Һ�ʼ��ԣ�˵�����������ᣬ���������ˮ�����Һ�ʼ��ԣ�ˮ�ⷽ��ʽΪCH3COO-+H2O?CH3COOH+OH-���ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-��
��2�����ݵ���غ��c��CH3COO-��+c��OH-��=c��Na+��+c��H+����
���������غ��c��Na+��=c��CH3COOH��+c��CH3COO-����
���Ե�c��CH3COOH��=c��OH-��-c��H+��=$\frac{1{0}^{-14}}{1{0}^{-9}}$mol/L-10-9mol/L=��1��10-5-1��10-9��mol/L��
�ʴ�Ϊ����1��10-5-1��10-9����
��3��CH3COONH4��Һ�����ԣ�˵��CH3COO-ˮ��̶Ⱥ�NH4+ˮ��̶���ͬ��CO32-ˮ��̶ȴ���CH3COO-�����Ԣ���Һ�ʼ��ԣ�
����Һ�����ԣ�
�ۢ���NH4+ˮ��̶���ͬ��������Һ�������ԣ�������NH4+Ũ�ȴ�����pH�ۣ��ܣ�
ͨ�����Ϸ���֪����ҺpH��С˳���Ǣ٢ڢܢۣ�
�ʴ�Ϊ���٢ڢܢۣ�
��4������狀��Ȼ�����ƣ�ֻ��笠����ӵ�ˮ�⣬��̼���ƺͰ�ˮ��Һ���ʼ��ԣ�ֻ��B���ϣ�
�ʴ�Ϊ��B��
��5����ͬѧ����ѡ�Լ��μӵ�Mg��OH��2����Һ�У�Mg��OH��2�ܽ⣬˵��������þ��笠����ӷ�Ӧ��ʹ������þ�ܽ⣬���ӷ���ʽΪMg��OH��2+2 NH4+�TMg2++2NH3•H2O��
�ʴ�Ϊ���ң�Mg��OH��2+2 NH4+�TMg2++2NH3•H2O��
��6��ͨ�����Ϸ���֪��������þ������Σ�����������������Σ���������γ�ȥ������þ����NH4NO3����CH3COONH4����ξ��ɣ�����������������ǿ���������þ������ǿ����Կ�����ǿ����Һ��ȥ������������NaOH��Һ�ȣ�
�ʴ�Ϊ��NH4NO3����CH3COONH4����ξ��ɣ���NaOH��Һ�ȣ�
���� ���⿼��������ʵĵ��뼰����ˮ�⣬Ϊ��Ƶ���㣬��������ڿ���ѧ����ʵ��̽�������ͷ���������ע�⣨3�����Ȼ�狀��������ҺpH��С�ȽϷ�����Ϊ�״��㣻����II�����ȷ�����������г�ȥ������þ�ķ�������Ŀ�ѶȲ���


 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ܼ������죬�õ��ľ������Ҳ�ϴ� | |
| B�� | ��ѹ���˿ɹ��˽�״������ҽ�Ϊ���� | |
| C�� | ��0.1 mol•L-1һԪ��BOH��Һ��pH=10������֪BOH��Һ��BOH�TB++OH- | |
| D�� | ��0.1 mol•L-1һԪ��HA��Һ��pH=3������֪NaA��Һ��A-+H2O?HA+OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
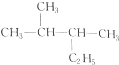 ��
��
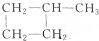 ��
��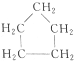
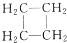 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3.36 L | B�� | 6.72 L | ||
| C�� | ����3.36 L����5.60 L | D�� | ����5.60 L����6.72 L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
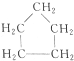
| A�� | 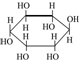 ���������Ϊͬϵ�� ���������Ϊͬϵ�� | |
| B�� | �߾���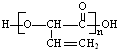 �� ��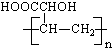 �ĵ�����ͬ �ĵ�����ͬ | |
| C�� | ��ϵͳ��������������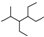 ������Ϊ2-��-3��4-�һ����� ������Ϊ2-��-3��4-�һ����� | |
| D�� | �����ʵ����ļ����������ȫȼ��ʱ����������ȣ����ɵ�CO2����Ҳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
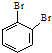 ��
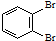
��
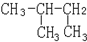 ��
��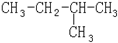
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢܢ� | B�� | �ڢۢݢ� | C�� | �ڢۢܢ� | D�� | ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH4+2O2$\stackrel{��ȼ}{��}$CO2+2H2O | B�� | CH2=CH2+Br2��CH2BrCH2Br | ||
| C�� | 2C2H5OH+2Na��2C2H5ONa+H2�� | D�� | 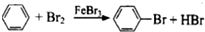 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ��ֻ�����ڷ���֮�� | |
| B�� | ��ѧ��ֻ����������֮�� | |
| C�� | ��ѧ�������ڵ�ԭ�ӻ�����֮��ǿ�ҵ������ | |
| D�� | ��ѧ�������ڵķ���֮��ǿ�ҵ������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com