
ij��ѧС��ͬѧΪ����֤�ճ��������û��ͷ�еĻ�ѧ�ɷ֣���KClO3��MnO2��S�ȣ������������ʵ�����̣���ͼ-1���� 
�Իش��������⣺
��1��ȼ�ŵĻ��ͼ-2����ʵ�飬���Թ����ܹ۲쵽 ���������֤�����ͷ�к�����Ԫ�ء�ͼ����Ͳ�������� ��
��2��Ϊ��֤�����ͷ�к�����Ԫ�أ�������ʵ�鲽���� ��
��3����ͬѧ���������ͷ��KClO3����һʵ�鷽����
�Լ�AΪ ������NaNO2��Ŀ���� ��
��4�����ʵ�飺������֤����D�к���MnO2��һ��ʵ�鷽������д���йط�Ӧ�Ļ�ѧ����ʽ�� ��
��1��KMnO4��Һ��ɫ����ɫ��dz��������ȷ������2�֣���
����˼��ȷ���ɣ���1�֣�
��2��ȡ������ҺC�����Թ��У��Ⱥ�����HNO3��AgNO3��Һ�����۲쵽��ɫ����������������֤�����ͷ�к�����Ԫ�ء���1�֡�3��
��3�������HNO3��1�֣�����ԭKClO3��1�֣�
��4��ȡ��������D����װ������H2O2���Թ��У��д������ݷų�˵������MnO2��2�֣���
2H2O2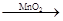 2H2O+O2����2�֣���������ȷ�𰸡�
2H2O+O2����2�֣���������ȷ�𰸡�
���������������1��������Ԫ��ȼ���������˶������������ʹKMnO4��Һ��ɫ����ɫ��dz������Ͳ����ʹȼ�ղ���������˳�������Թܡ�
��2����Ԫ��ȼ������Ҫ�������Ȼ������壬ֻҪȡ������ҺC�����Թ��У��Ⱥ�����HNO3��AgNO3��Һ�����۲쵽��ɫ����������������֤�����ͷ�к�����Ԫ�ء��൱���������ӵļ��顣
��3��KClO3��ǿ�������ԣ������Ի����£���������HNO3��Ҫ���ữ�������������н�ǿ�Ļ�ԭ�ԣ����Ի�ԭKClO3Ϊ�����ӡ�
��4����֤�Ƿ���MnO2��������ʵ������ȡ�����ķ�����Ҳ������Ϊ����ȡ��������D����װ������H2O2���Թ��У��д������ݷų�˵������MnO2����ط�Ӧ 2H2O2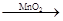 2H2O+O2��
2H2O+O2��
���㣺������ʵ��̽����˼·��Ԫ�ػ������еĻ���֪ʶ��


 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ���ҿ������Ҵ���Ũ������170 ������ϩ����ѧ��Ӧԭ����CH3CH2OH CH2=CH2����H2O������Ũ�������ǿ�����ԣ��丱�����ж�������Ͷ�����̼�ȡ�ijͬѧ����������Ϣ��ʵ��Ŀ��ѡ������ʵ��װ�����ʵ��̽��(ÿ��װ�ö������ɸ�)��
CH2=CH2����H2O������Ũ�������ǿ�����ԣ��丱�����ж�������Ͷ�����̼�ȡ�ijͬѧ����������Ϣ��ʵ��Ŀ��ѡ������ʵ��װ�����ʵ��̽��(ÿ��װ�ö������ɸ�)�� 
��1����֤�Ҵ���Ũ�����Ϸ�Ӧ�������ж�������Ͷ�����̼��
��ѡ���Ҫװ������˳��a�� ��f��g��e��d��b(��ӿ����)��
��ȷ�ϻ���������ж�����̼���ڵ�ʵ��������
��2�����Ʊ�1,2�����������װ��ΪA��E��C��D��
��D�еķ�Ӧ����Ϊ
�ڷ���1,2����������IJ�����ʹ�õIJ���������_____________________________
����ͬѧ������A��E֮������Gװ�ã���������
��3���Ķ�����֪�������Ը��������Һ��������ϩ���ɶ�����̼����������Ʒ�����֤������ʵ�ԣ�
��װ�õ�����˳��A��____________________(��װ�����)��
����֤���������Ϸ�����ʵ��ʵ��������_______________________��
��4��Ϊ��̽����ϩ����ˮ�ķ�Ӧ��ȡ����Ӧ���Ǽӳɷ�Ӧ����ͬѧ��������ʵ�飺�ٲⶨDƿ����ˮ��pH�� �ڽ���������ϩͨ��D����Һ��ȫ��ɫ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��Ƴ������������ƹ��գ������ŷŵķ�ˮ�к��еľ綾CN-���ӣ����������ƹ�����������������Ƶķ�ˮʱ�����ڴ���TiO2�����£�����NaClO��CN-����������OCN-���������������¼�����NaClO������N2��CO2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬��֤��������������Ч�ԣ����ⶨCN-�������İٷ��ʡ���Ũ����CN-���ӵ���ˮ�����NaClO��Һ�Ļ��Һ��200mL������CN-��Ũ��Ϊ0.05mol��L-1��������У�������Ƥ����һ��ʱ�����Ƥ���ͻ�����ʹ��Һȫ���������У��رջ������ش��������⣺
��1�����з�Ӧ�����ӷ���ʽΪ ��
��2���������ɵ������N2��CO2�⣬���и�����HCl��Cl2�ȣ�����ʵ����ͨ���ⶨ������̼������ȷ����CN-�Ĵ���Ч��������м���ij����Լ���___________������ĸ��
| A������ʳ��ˮ | B������NaHCO3��Һ | C��ŨNaOH��Һ | D��Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
MgSO4?7H2O��ҽҩ�ϳ�����кҩ����ҵ�Ͽ����ȼҵ�е�һ������Ϊԭ����������֪һ�������к���þ���ơ����������̵Ĺ����κ�̼���εȳɷ֡�����Ҫ�������£�
��H2SO4������ҺPH��1~2��H2SO4������ ��
��2����NaClO��Һ��PH=5~6���������5~10���ӣ�����2����Ҫ����MnO2���������������������������Ļ�ѧʽ�ֱ�Ϊ �� ������NaClO��Һ��MnSO4ת��ΪMnO2�����ӷ���ʽΪ ��
��3��������Һ�����Ƿ���Fe3+��ʵ�鷽���� ��
��4��������X���ǽ���Һ �� ������ϴ�ӣ����õ�MgSO4?7H2O���塣
��5��ȷ�����Ƶõ���Ʒ5g,��200mLˮ�ܽ⣬����2 mol?L��1������5mL���ڲ��Ͻ����µμ�����2 mol?L��1 BaCl2��Һ����ַ�Ӧ�ó���4.46g������Ʒ����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ѧ����������������Ϊ�Ҵ���ˮ����ϩ�Ĵ������������ʵ�顣���ұ���ʾ�����ͷ�Ӧ����������ƿ�м���һ����P2O5����ע��95%���Ҵ��������ȣ��۲�����
| ʵ�� | P2O5 /g | 95%�Ҵ���/ mL | ���ȷ�ʽ |
| ʵ��1 | 2 | 4 | �ƾ��� |
| ʵ��2 | 2 | 4 | ˮԡ70�� |
| ʵ�� | ʵ������ | ||
| ����ƿ | �ռ�ƿ | �Թ� | |
| ʵ��1 | �ƾ�����ʱ�����̷ų������İ�������ʼ�����ݲ��������þƾ��Ƽ���ʱ�����ݼӿ����ɲ����ڣ�����ճ��״Һ�塣 | ����ɫҺ�� | ��Һ��ɫ |
| ʵ��2 | �ƾ�����ʱ���������������ɣ�����ˮԡ����ʱ�����������ݣ���Ӧһ��Сʱ����Ӧƿ������ճ��״Һ�� | ����ɫҺ�� | ��Һ����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ����С���Ժ���Ϊԭ�ϻ��������ˮ���������Ȼ�̼���е�76.80C��Ϊ�ܼ�����ӵ�ˮ����ȡ���������������ͼ��ʾ��
��ش��������⣺
��1�������ٵ�����Ϊ �� ��
��2�����������õ���������д��ʵ������ȡ������Ӧ�����ӷ���ʽ ��
��3���ⵥ�ʵ�ˮ��Һ�м���CCl4�����ú۲쵽�������� ��
��4���������У������ⵥ�ʵ�ˮ��Һ��CCl4�ڷ�Һ©���л�ϡ���ҡ�ȷ�������̨����Ȧ�ϣ���Һ©�����¶˼��촦�����ڳнӵ��ձ��ڱ��Ͼ��ú�����IJ��������� ��
��5���Ӻ�����л��ܼ��о������������ȡ��ͻ����л��ܼ�����ʵ����Ҫ����Ҫ�����������ƾ��ơ��ձ����¶ȼơ���ƿ�⣬����Ҫ ��ʵ�����¶ȼ�ˮ��������λ��Ϊ ����ƿ���ռ������ʵ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ�����Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�Ϊԭ����ȡCaCl2��H2O��CaO2����Ҫ�������£�
��1�������Լ�X��������ҺpHΪ���Ի������Գ�ȥ��Һ��Al3+��Fe3+����������Ҫ�ɷ���___________���Լ�X����ѡ�����е�________�����ţ���
| A��CaO | B��CaCO3 | C��NH3��H2O | D��Ba(OH)2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ͼɵ�ؽ��뻷�����������һϵ�е��»����°����±��Σ�����ϵ������Ҫ��ͭñ����Cu��Zn����п�ǡ���Ƭ��ʯī������MnO2��NH4Cl�����ԷϾɵ�ؽ�����Դ�������Ĺ����������£�
��1������A������Ϊ �������ijɷ�Ϊ ��
��2���������60����ˮ�ܽ⣬Ŀ����Ϊ�˼ӿ��ܽ����ʣ�����������¶Ȳ���̫�ߣ���ԭ���� ��
��3��ͭñ�ܽ�ʱ����H2O2��Ŀ���ǣ��û�ѧ����ʽ��ʾ�� ��ͭñ�ܽ���ȫ���轫��Һ�й�����H2O2��ȥ����ȥH2O2�ļ�㷽���� ��
��4������п�̵�صĵ����ΪKOH���ܷ�ӦΪ��Zn+2MnO2+2H2O===2MnOOH+Zn(OH)2���为���ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com