
(16��)
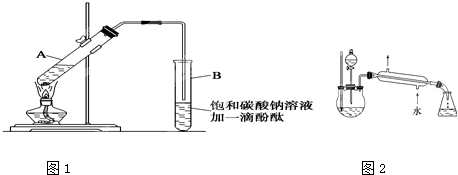
��1��ʵ���������Ľ����Ʊ����� �У�ȡ��ʱ�õ�����������Ʒ�� , ʣ�����Ӧ ����һС����Ͷ�뵽����ͭ��Һ�У���Ӧ�����ӷ���ʽΪ ���۲쵽������Ϊ (��д���)��
a���Ƹ���Һ�����Ĵ��ζ� b�����ڳ���һ��������С��
c����Һ������ɫ�������� d����Һ���к�ɫ��������
��2��ʵ�����������Ļ�ѧ��Ӧ����ʽΪ_________________________________������Ϊ�ж����壬��������������Һ����β������������ɸ����ӷ���ʽ �� ��ԭ���������ڹ�ҵ�� ��
��3���������ʼ������ᷴӦ��������Ӧ����
a. Al b. Mg c. CH3COONH4 d. NaHCO3 e. Al2O3
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g/cm3�� |
| �Ҵ� | -117.0 | 78.0 | 0.79 |
| ���� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���ᣨ98%�� | - | 338.0 | 1.84 |
| Ũ���� |
| ���� |
| Ũ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�������и���8���¿������Ծ����������� ���ͣ������
(16�֣���һƿ�������Һ�����п��ܺ���NH4+��K+��Na+��Mg2+��Al3+��Fe3+��Ba2+��Cl����I����NO3����CO32����SO42���еļ��֡�ȡ����Һ��������ʵ�飺
(1)��pH��ֽ�������Һ��ǿ���ԣ��ų� ���ӵIJ����ڡ�
(2)ȡ��������Һ����������CCl4������������ˮ������CCl4����Ϻ�ɫ�������ų�
���Ӳ����ڡ�
(3)��ȡ������Һ����������μ���NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ��������ɣ�����ų� ���Ӳ����ڡ�ȡ���ּ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������˵�� ���Ӵ���
(4)��ȡ��������������Һ�������м���Na2CO3��Һ���а�ɫ�������ɣ������ų� ���Ӳ����ڡ�
(5)��������ʵ����ʵȷ��������Һ�п϶����ڵ������� �������ܿ϶��Ƿ���ڵ������� ��д����(3)�����������ӷ�Ӧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����������ݶ��и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��16�֣�ÿ��2�֣�ij�����Ĺ�ҵ��ˮ�к��д�����FeSO4���϶��CuSO4������Na2SO4��Ϊ�˼�����Ⱦ�����Ϊ���������ƻ��Ӹ÷�ˮ�л������������ͽ���ͭ������� ��������ͼ����ɻ�������������ͭ��ʵ�鷽�������ɹ�ѡ����Լ�Ϊ���ۡ�ϡH2SO4��
��������ͼ����ɻ�������������ͭ��ʵ�鷽�������ɹ�ѡ����Լ�Ϊ���ۡ�ϡH2SO4�� NaOH��Һ���Լ���
NaOH��Һ���Լ���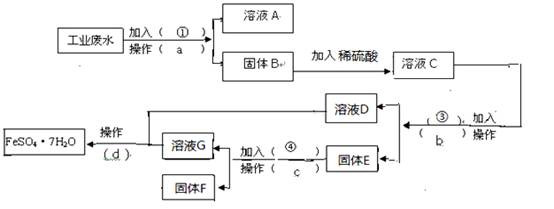 ��1������a������Ϊ ������Ҫ�IJ�������Ϊ ____��
��1������a������Ϊ ������Ҫ�IJ�������Ϊ ____��
��2������E�ijɷ�Ϊ  ____��������Լ���Ϊ __�������Ļ�ѧ����ʽΪ ��
____��������Լ���Ϊ __�������Ļ�ѧ����ʽΪ ��
��3�������Լ��ٵ�Ŀ ���� ____________��
���� ____________��
��4������ҺD����ҺG�еõ�FeSO4.7H2O����IJ���Ϊ ����ȴ�ᾧ ��
��ϴ�ӡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������ʡ��������е���У����߶���ѧ����ĩ���� ���ͣ������
(16��)��֪��RCH��CH2 RCH2COOH����ͼ��ʾ�л���A��B��C��D��E��F��X��Y֮���ת���ϵ��
RCH2COOH����ͼ��ʾ�л���A��B��C��D��E��F��X��Y֮���ת���ϵ��

���У�X��Y��Ϊͬ���칹�壬F��C��Ϊͬϵ�E��D��Ϊͬϵ�B�к�֧�����Һ˴Ź������ײ����3�����շ壬���6��1��1��A���ܶ��ڱ�״����Ϊ1.25g/L��A��B��X��Y�ķ���ʽ������CnH2nO0. 5n-1
��1��B�Ľṹ��ʽΪ ��Y�Ľṹ��ʽΪ
��2��C��D����X�Ļ�ѧ��Ӧ����Ϊ
A���ӳɷ�Ӧ B����ȥ��Ӧ
C��ȡ����Ӧ D��������Ӧ
��3��ʵ������ȡA�Ļ�ѧ��Ӧ����ʽΪ ��
��4��ʵ������װA�ķ���װ��ʱ���õ��IJ���������Ҫ�оƾ��ơ�������
��5���ռ�A��װ���� (�����)
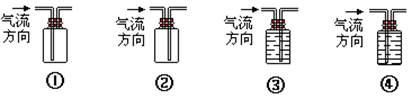
��6��ʵ������ȡAʱ�������¶ȹ��߶������д̼�����ζ������e�����һ����ʵ������֤����װ�������ɵ�A�л�ԭ�ԣ���ʹ������������ͨ��������Һ�е�һ�ֻ��֣�����ѡ�Լ���˳��Ϊ (����ţ��ɲ�ȫѡ��Ҳ�����ظ�ʹ��)
��Ʒ����Һ ����ˮ
������������Һ �����Ը��������Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com