
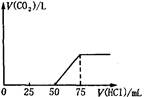
| A��A��Һ������Na2CO3��NaHCO3���ʵ�����Ϊ1��1 |
| B��A��Һ������NaOH��Na2CO3���ʵ�����Ϊ1��1 |
| C��ԭNaOH��Һ���ʵ���Ũ��Ϊ0.075mol/L |
| D��ͨ��CO2�����ڱ���µ����Ϊ56mL |
| ��Һ�����ʵijɷ� | Na2CO3��NaOH | Na2CO3 | Na2CO3��NaHCO3 | NaHCO3 | NaHCO3 |
| ����Һ����μ���ϡ���ᷢ���ķ�Ӧ | �����Ǣݢۢ� | �����Ǣۢ� | �����Ǣۢ� | �� | �� |
| V1��V2�Ĺ�ϵ | V1��V2 | V1=V2 | V1��V2 | V1=0��V2��0 | V1=0��V2��0 |


 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��������ȡ�����Ҫװ������:
��������ȡ�����Ҫװ������: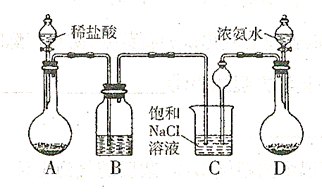
 ,�����D������(ֻ��һ�� ��Cװ��������©���������� ��
,�����D������(ֻ��һ�� ��Cװ��������©���������� �� ������ܺ��е������� ���ø�
������ܺ��е������� ���ø�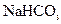 ������ȡ����ʱ���е����ʲ���Ӱ�촿��Ĵ��ȡ�ԭ����
������ȡ����ʱ���е����ʲ���Ӱ�촿��Ĵ��ȡ�ԭ���� 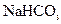 ��ȡ����Ļ�ѧ����ʽ ��
��ȡ����Ļ�ѧ����ʽ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
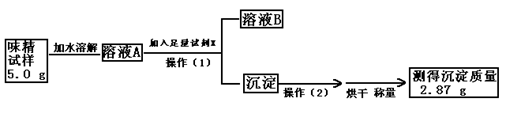 [
[ �� ��
�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Т� | B��ֻ�Т� | C��ֻ�Тۢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 Z+ H2O �� Y + 02�� �� X + Ca(OH)2�� Y + CaCO3��
Z+ H2O �� Y + 02�� �� X + Ca(OH)2�� Y + CaCO3���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ol��L��1Na2CO3��Һ�����в�����ȷ���� ( )
ol��L��1Na2CO3��Һ�����в�����ȷ���� ( )| A����ȡ10.6 g��ˮ̼���ƣ�����100 mL����ƿ�У���ˮ�ܽ⡢���� |
| B����ȡ10.6 g��ˮ̼���ƣ�����100 mL����ˮ�����衢�ܽ� |
| C��ת��Na2CO3��Һʱ��δ�ò�����������ֱ�ӵ�������ƿ�� |
| D�����ݺ�����ƿ����������ת��ҡ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
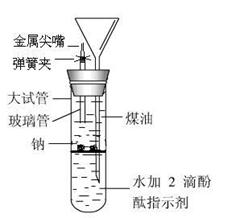
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com