
��ȸʯ����Ҫ�ɷ�ΪCu2(OH)2CO3���������������������������������ʵ
�����Կ�ȸʯΪԭ���Ʊ�CuSO4��5H2O���������ͼ��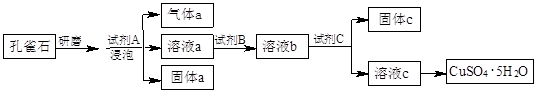
��1����ĥ��ȸʯ��Ŀ���� �����ݿ�ȸʯ���Լ�A��ѡ�ù�����ϡ���ᣬ�����a�� (�ѧʽ)��
��2���Լ�B��Ŀ���ǽ���Һ�е�Fe2+ת��ΪFe3+�����Լ�B��ѡ�� ������ţ���
A������KMnO4��Һ B��˫��ˮ C��Ũ���� D����ˮ
��Ӧ�����ӷ���ʽΪ�� ��
��3���Լ�C���ڵ�����ҺpH��ʹFe3+ת��Ϊ�������Է��롣���Լ�C��ѡ�� ������ţ���
A��ϡ���� B��NaOH��Һ C����ˮ D��CuO
����C�Ļ�ѧʽΪ ��
��4��1 mol����ͨ�����ȵ�Cu2(OH)2 CO3���Բ���1.5 mol����ͭ����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��������Fe(OH)3��Ksp=1��10-39����Ҫ����Һ�е�Fe3+ת��ΪFe(OH)3������ʹ��Һ��c(Fe3+)������1��10��3 mol��L�����轫��Һ������pH= ��
��1������4�֣��������������1�֣���������Ӧ���ʣ�1�֣�[������˼����]
SiO2��2�֣�
��2������4�֣�B��2�֣� 2Fe2++2H++H2O2=2Fe3++2H2O��2�֣���δ��ƽ1�֣�дΪ��ѧ����ʽ2FeSO4 + H2SO4 + H2O2 = Fe2(SO4)3 + 2H2O����ȷ1�֣�
��3������4�֣�D��2�֣� Fe(OH)3��2�֣�
��4������3�֣�3Cu2(OH)2CO3 + 4NH3 6Cu + 3CO2 + 9H2O +2 N2����ѧʽ1�֡���ƽ��1�֣�����1�֡���
6Cu + 3CO2 + 9H2O +2 N2����ѧʽ1�֡���ƽ��1�֣�����1�֡���
��5������2�֣�2
���������������1����ĥ��ȸʯ��Ŀ����Ϊ���������ʸ��죬���Դ�Ϊ���������Ӵ�������ӿ췴Ӧ���ʡ������ܹ����в��ܵĹ���Ϊ����SiO2��
��2����Fe2+ת��ΪFe3+ Ӧ�ü������������Ҳ��ܴ����������ӣ�������˫��ˮ��ѡB����Ӧ�����ӷ���ʽΪ��2Fe2++2H++H2O2=2Fe3++2H2O��
��3����ΪĿ���Ʒ��CuSO4��5H2O���壬���Ե���pHֵʹ�����ӳ���Ӧѡ�õ��Լ����ܴ����������ӣ�����ѡ�ù���CuO�����Գ�����cΪFe(OH)3 ������CuO��
��4����ʽ̼��ͭ��ͨ�백������ͭ�������ɣ�˵��ͭ����ԭ��NH3����ԭ������Ϊ1mol������ԭ�õ�1.5molͭ���ʣ�����ͭ+2��0�ۣ���֪ת�Ƶ���3mol����ʧ�����غ㣬����NH3ʧȥ����ҲΪ3mol�����Կ��Ʋ��NH3������Ϊ��N2�����Կ��Ե�֪�������ﻹ��CO2��H2O�������г���Ӧ��NH3+ Cu2(OH)2 CO3��Cu+ N2+CO2+H2O��Ȼ�����������ԭ��Ӧ����ʽ��ƽ�ã�3Cu2(OH)2CO3 + 4NH3 6Cu + 3CO2 + 9H2O +2 N2 ��
6Cu + 3CO2 + 9H2O +2 N2 ��
��5������Fe(OH)3�ܽ�ƽ��Fe(OH)3 (s) Fe3+ (aq)+3 OH- (aq)�ɵ�Ksp ="c(" Fe3+ ) ��c3(OH- )= 1��10-39 ������c(Fe3+)=1��10��3 mol��L���빫ʽ�У������c(OH- )= 1��10-12 ������c(H+ )="Kw/" c(OH- )= 10-2 ������pH=2��
Fe3+ (aq)+3 OH- (aq)�ɵ�Ksp ="c(" Fe3+ ) ��c3(OH- )= 1��10-39 ������c(Fe3+)=1��10��3 mol��L���빫ʽ�У������c(OH- )= 1��10-12 ������c(H+ )="Kw/" c(OH- )= 10-2 ������pH=2��
���㣺���⿼����ǻ������������⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������ѧ����������Ϊ���ü�ͥ������Ʒ������ѧʵ�飬�Ի�ѧ����ѧϰ���о��Ļ��ij��������һ��NaCl��Na2CO3��10H2O��NaHCO3�Ļ���ijͬѧ�������ʵ�飬ͨ��������Ӧǰ��C��Dװ�������ı仯���ⶨ�û�����и���ֵ�����������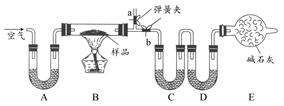
(1)ʵ��ʱ��B�з����ķ�Ӧ�Ļ�ѧ����ʽΪ ��
(2)װ��C��D��ʢ�ŵ��Լ��ֱ�Ϊ��C ��D ��(��ѡ�Լ�Ϊ��Ũ���ᡢ��ˮCaCl2����ʯ��)
(3)Eװ���е����������� �����ڸ�ʵ���е���Ҫ������ ��
(4)����Aװ�û���ʢ��NaOH��Һ��ϴ��ƿ�����õ�NaCl������ (�ƫ�ߡ���ƫ�͡�����Ӱ�족)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʽ̼��ͭ�ɱ�ʾΪ��xCuCO3��yCu��OH��2��zH2O,�ⶨ��ʽ̼��ͭ��ɵķ����ж��֣�Cu�����ԭ��������63��5�ƣ���
��1���ֲ���������ԭ������ش��������⣺
��֪xCuCO3��yCu��OH��2��zH2O��������Ӧ�Ļ�ѧ����ʽΪxCuCO3��yCu��OH��2��zH2O+
��x+y��H2 ��x+y��Cu+xCO2+��x+2y+z��H2O
��x+y��Cu+xCO2+��x+2y+z��H2O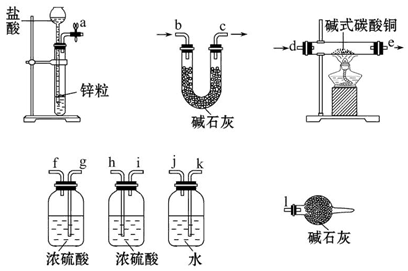
��ʵ��װ�������������������Ӷ��ɣ������������������˳���ǣ����������ӿ���ĸ��ţ���
��a������ ���� ������ ���� ������ ���� ������ ���� ������ ���� ������l����
�ڳ�ȡ23��9 gij��ʽ̼��ͭ��Ʒ����ַ�Ӧ��õ�12��7 g ���������4��4 g������̼��7��2 gˮ������Ʒ�Ľᾧˮ����Ϊ_____g����ѧʽΪ_____________��
��2��ijͬѧ�Ե���������������������ȫ�����������ⶨ��ʽ̼��ͭ����ɣ�����Ϊ�Ƿ���У���˵������_________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������������ڿ�������Ѹ�ٱ������������������������Ҫ�۲쵽��������������ɫ������Ϊ���ѡ�ij�о���ѧϰС���ͬѧ�������ۺ���Ƴ��������ܽϳ�ʱ��۲�Fe(OH)2������ɫ��װ�ã���ͼ��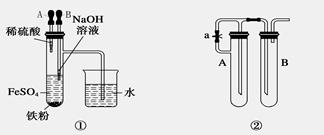
1.����װ�â٣�Ҫ��ﵽʵ��Ŀ�ģ�Ӧ�ȼ���ͷ�ι�_______���A����B����
2.����װ�âڣ������Լ�ΪNaOH��Һ����м��ϡ���ᣬ
��1���Թ�A�м�����Լ���_________�Թ�B�м�����Լ���_______
��2��Ϊ���Ƶ�Fe(OH)2��ɫ�����������������²����������Թ�A��B�м����Լ���Ȼ��_____�������رա���ֹˮ�У��������ӣ���������ʵ�鲽����____________________
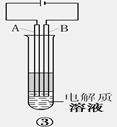
3.װ�â�ʱ���õ��ԭ�����ﵽʵ��Ŀ�ĵġ�
��1���缫������������Fe����̼����C����A��ӦΪ_______������ϵ�Ԫ�ط��ţ�
��2���������Һ��ѡ��__________����ѡ����ţ�
A.NaCl��Һ B.CuSO4��Һ C.Na2SO4��Һ D.NaOH��Һ
��3����ʵ����ѡ��һ���л������������������ã����л��������__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������䡢��ˮ��Ӧ����������ˮ������Ӧ��ʵ��װ����ͼ��ʾ����Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ���������ˮ������Ӧ��ʵ�顣��ش�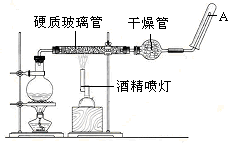
��1��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ��ǰ���������װ�ý��еIJ�����________��ʵ�鿪ʼʱӦ�ȵ�ȼ ����ƾ��ơ��ƾ���ơ�����ʵ�����ʱӦ��Ϩ�� ����ƾ��ơ��ƾ���ơ�����
��3��Բ����ƿ��ʢ��ˮ����װ�����Ⱥ����Ҫ������ ����ƿ��Ӧ���ȷ��� ���������� ���������ʢװ��������________��
��4����Ӳ�ʲ�������ȴ��ȡ�������еĹ�����������ϡ�������ҺB��ȡ������ҺB�μ� ��Һ���� ����ʵ��������˵����ҺB�к���Fe3+��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС�飬Ϊ��̽���������Ƶ�ǿ�����ԣ��������ͼ��ʵ��װ�á�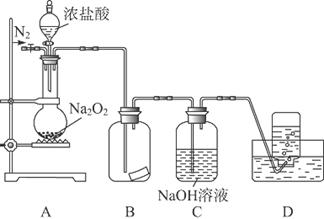
ʵ�鲽�輰�������£�
�ټ��װ�������Ժ�װ��ҩƷ������������
�ڻ���ͨ��һ������N2��װ��D���Ӻ�(����ĩ��δ���뼯��ƿ��)������Բ����ƿ�л����μ�Ũ���ᣬ��Ӧ���ң���������ɫ���塣
��һ��ʱ�������ĩ�����뼯��ƿ���ռ����塣װ��D���ռ�����ʹ�����ǵ�ľ����ȼ����ɫ���塣
�ܷ�Ӧ�����رշ�Һ©���Ļ�������ͨ��һ������N2����װ����������ɫ��
�ش��������⣺
��1��װ��B�е�ʪ��ĺ�ɫֽ����ɫ��֤��A�з�Ӧ��________(�ѧʽ)���ɡ���B�иķ�ʪ��ĵ���KI��ֽ����ƾ��ֽ������������֤���������ۣ��������ӷ���ʽ˵��ԭ��________________��
��2��װ��C��������_________________________________________________________��
��3����ͬѧ��ΪO2��Na2O2�������е�HCl��ԭ���á���ͬѧ��Ϊ�˽��۲���ȷ�������ܵ�����Ϊ��_________________________________________________________________��
��______ __��
��4��ʵ��֤����Na2O2��������HCl��Ӧ����ɲ���ƽ�û�ѧ����ʽ��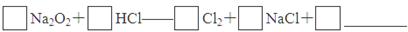
�÷�Ӧ________(��ܡ����ܡ�)����ʵ���ҿ�����ȡ������Cl2��������___________________________________________________________________________________________________________________________________(Ҫ����Ҫ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
һѧϰС��������ͼ��ʾװ�ã���ij������Fe�ķ�ͭм����ͭ�����IJⶨ����̽���������Ʊ�����ͭ��Һ��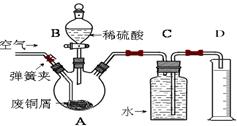
��1����A�м���10g��ͭм��Ʒ���رյ��ɼУ���B������Aע������ϡ�����رա��ٴ�ʱװ��C�в����������� ��
�ڷ�Ӧ���е�A�в��ٲ�������ʱ����C���ռ���448mL���ѻ���ɱ�״�������壬��÷�ͭм��ͭ�������ٷֺ���Ϊ ��
��2��������ʹCu��ȫ�ܽ�IJ����ǣ�
�� ���C��Dװ�ú��ɼ�
��____________________________________________________________________
��3��ΪʹA�й���ӿ��ܽ����ʣ����·������ú������� ��
a.��Aװ�ü��ȣ�b.��A�ڼ�������Fe2O3��c.��A�ڼ�������CuO��d.�������ͨ������e.��A�ڼ�������FeSO4��f.��A�ڼ�������H2O
��4����A����Һ�����ձ��ڣ�����Cu2(OH)2CO3���������pH=4ʱ����Һ����Ԫ�ر���ȫ���������˺ú��ɫ����������ͭ��Һ��
�ٴ˹��̵����ӷ�Ӧ����ʽ�� ��
�ڼ�����Һ����Ԫ���Ƿ���ȫ������õķ�����___________
a.ȡ�����Թܡ��μ�KSCN��Һ
b.ȡ�����Թܡ��μ�����KMnO4��Һ
c.ȡ�����Թܡ�ֽ�ϲ������ ��KSCN��Һ��
��5����ͬѧ��Ϊ�����Բ��ò������巨�����ͭм��ͭ�������ٷֺ��������������������дΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ӳ�ʲ������ǻ�ѧʵ���о���ʹ�õ�һ�����������������ʵ�飨�̶�װ���ԣ����ش����⡣
������ʵ�飺��ͼ��ʾ����Ũ�������װ��Na2SO3�����������һ��ʱ���a��b��c��������仯���±�������д���еĿհף�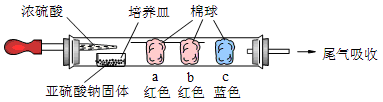
| ���� | �����ϵμӵ��Լ� | ʵ������ | ���ͺͽ��� |
| a | | �����ף��Ⱥ��ָֻ���ɫ |  |
| b | ����̪��NaOH��Һ | �����Ϊ��ɫ | ���ӷ���ʽ�� |
| c | | �����Ϊ��ɫ | ���ۣ���������� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
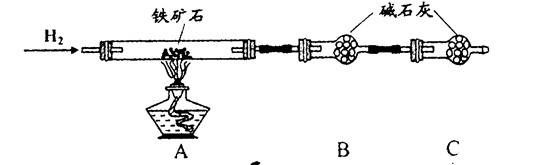
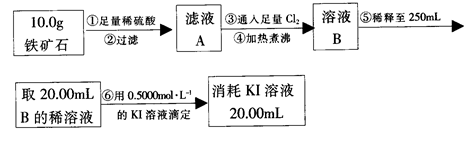
����ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ���H2SO4��Ӧ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
������ʯ�к������IJⶨ��
�� ����ͼ��װ���������װ�õ������ԣ�
�� ��5.0g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��Ϊ��ʯ��(���ͼʾ���г�����ʡ��)
�� ����˵����ܿڴ����ϵػ���ͨ��H2����Cװ�ó��ڴ�H2�鴿��ȼA���ƾ���
�� ��ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ����������ȫ��ȴ��
��1��װ��C������Ϊ �������� ��������������
��2����ķ�Ӧ��װ��B����1.35g��������ʯ�����İٷֺ���Ϊ�������� ��
������ʯ�к������IJⶨ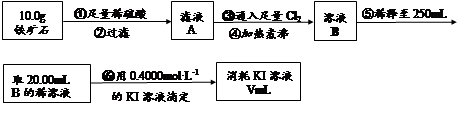
��3����������������������������������������������������������� ��
��4����������õ��IJ����������ձ�������������ͷ�ιܡ������� ���� ��
��5�������йز���IJ�����˵����ȷ���������������������� �������� ��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b���ζ������п����õ�����Һ��Ϊָʾ��
c���ζ���������ˮϴ�Ӻ����ֱ��װҺ
d����ƿ����Ҫ�ô���Һ��ϴ
e���ζ������У��۾�ע�ӵζ�����Һ��仯
f���ζ�������30s����Һ���ָ�ԭ������ɫ�ٶ���
��6�����ζ�����������0.4000mol��L?1KI��Һ25.00ml��������ʯ�����İٷֺ���Ϊ�� ����
��7���ɢ���������������ʯ������������Ļ�ѧʽΪ����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com