
���������䡢��ˮ��Ӧ����������ˮ������Ӧ��ʵ��װ����ͼ��ʾ����Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ���������ˮ������Ӧ��ʵ�顣��ش�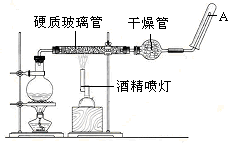
��1��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ��ǰ���������װ�ý��еIJ�����________��ʵ�鿪ʼʱӦ�ȵ�ȼ ����ƾ��ơ��ƾ���ơ�����ʵ�����ʱӦ��Ϩ�� ����ƾ��ơ��ƾ���ơ�����
��3��Բ����ƿ��ʢ��ˮ����װ�����Ⱥ����Ҫ������ ����ƿ��Ӧ���ȷ��� ���������� ���������ʢװ��������________��
��4����Ӳ�ʲ�������ȴ��ȡ�������еĹ�����������ϡ�������ҺB��ȡ������ҺB�μ� ��Һ���� ����ʵ��������˵����ҺB�к���Fe3+��
��1��3Fe+4H2O(g) Fe3O4+4H2����2�����װ�õ������ԣ��ƾ��ƣ��ƾ���ƣ�
Fe3O4+4H2����2�����װ�õ������ԣ��ƾ��ƣ��ƾ���ƣ�
��3��Ϊʵ���ṩˮ���������Ƭ����ֹ���У���ʯ�ң�������Ҳ���֣�����4��KSCN����Һ���ɫ������������Ҳ���֣���
���������������1�������£�����ˮ������Ӧ������������������������ѧ����ʽΪ3Fe+4H2O(g) Fe3O4+4H2����2��ʵ��ǰ���������װ�ý��еIJ����Ǽ��װ�õ������ԣ�ʵ�鿪ʼʱ��Ҫ���ž�װ���еĿ�����Ӧ�ȵ�ȼ�ƾ��ƣ�ʵ�����ʱ��Ӧ�Dz�����ˮ��������ȴ��Ӧ��Ϩ��ƾ���ƣ� ��3��Բ����ƿ��ʢ��ˮ����װ�����Ⱥ����Ҫ������Ϊʵ���ṩˮ��������ƿ��Ӧ���ȷ������Ƭ���������Ƿ����У��������ʢװ�������Ǽ�ʯ�ҡ���4������Fe3+�ļ��飻��Ӳ�ʲ�������ȴ��ȡ�������еĹ�����������ϡ�������ҺB��ȡ������ҺB�μ�KSCN��Һ������Һ���ɫ����˵����ҺB�к���Fe3+��
Fe3O4+4H2����2��ʵ��ǰ���������װ�ý��еIJ����Ǽ��װ�õ������ԣ�ʵ�鿪ʼʱ��Ҫ���ž�װ���еĿ�����Ӧ�ȵ�ȼ�ƾ��ƣ�ʵ�����ʱ��Ӧ�Dz�����ˮ��������ȴ��Ӧ��Ϩ��ƾ���ƣ� ��3��Բ����ƿ��ʢ��ˮ����װ�����Ⱥ����Ҫ������Ϊʵ���ṩˮ��������ƿ��Ӧ���ȷ������Ƭ���������Ƿ����У��������ʢװ�������Ǽ�ʯ�ҡ���4������Fe3+�ļ��飻��Ӳ�ʲ�������ȴ��ȡ�������еĹ�����������ϡ�������ҺB��ȡ������ҺB�μ�KSCN��Һ������Һ���ɫ����˵����ҺB�к���Fe3+��
���㣺���������仯���������ʵ�顣


 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ͭ����������������ʹ�õĽ���֮һ���ڻ�ѧ��Ӧ��ͭԪ�ؿɱ���Ϊ0��+1��+2�ۡ�
��1���������ż������м��أ������������Ϊͭ�����������ࣨCuSO4��������Ӧ����ͭ����д���÷�Ӧ�����ӷ���ʽ ��
��2����ԭ��غ͵����У�ͭ�������缫�������й�˵����ȷ����
| A��пͭԭ�����ͭ������ | B���õ�ⷨ����ͭʱ��ͭ������ |
| C���ڶƼ��϶�ͭʱͭ���Դ�������� | D��ͭ������ʱ��һ���ܽ� |
|
 ��ʵ����̡�
��ʵ����̡��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��þ�Ͻ��ѳ�Ϊ�ִ����졢������������ҵ����Ҫ���ϡ��о���ѧϰС�����λͬѧ��Ϊ�ⶨij��þ3%��5%����þ�Ͻ�(��������Ԫ��)��þ������������������������ֲ�ͬʵ�鷽������̽������д���пհס�
��̽��һ��ʵ�鷽������þ�Ͻ� �ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
ʵ�鲽�裺
(1)��ȡ5.4 g��þ�Ͻ��ĩ��Ʒ��Ͷ��V mL 2.0 mol��L-1 NaOH��Һ�У���ַ�Ӧ��NaOH��Һ�����V�ݣߣߣߣߣߣߡ�
(2)���ˡ�ϴ�ӡ�����������塣�ò�������δϴ�ӹ��壬���þ�������������ߣߣߣߣߣ�(�ƫ�ߡ���ƫ�͡�)��
��̽������ʵ�鷽������þ�Ͻ� �ⶨ������������ʵ��װ�ã�
�ⶨ������������ʵ��װ�ã�
�������ۣ�
(1)ijͬѧ�����ʵ��װ�ò������ƣ�Ӧ��A��B֮������һ�������������װ�á��������ǣ��ߣߣߣߣ�(���Ҫ������Ҫ��)��
(2)Ϊʹ�ⶨ��������ܾ�ȷ��ʵ����Ӧע���������(д������)��
�٣ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�ڣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��̽������
ʵ�鷽��������x g��þ�Ͻ��ĩ��������ͼ��ʾװ�õĶ��Ե��Ȱ��ϣ�ͨ��ʹ�������ա�
�������ۣ�
(1)������þ��������������ʵ���л���ⶨ�������ǣߣߣߣߣߣߡ�
(2)���ÿ���(������CO2)����O2����ʵ�飬�Բⶨ����к�Ӱ��? �ߣߣߣߣ�(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧ��ȤС��Ϊ̽������Ũ���ᷴӦ,�����ͼ1��ͼ2��ʾװ�ý���ʵ�顣
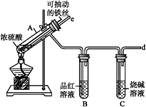
��������ͼ1���������������� ��ͼ2
(1)�Ƚ���ʵ��װ��,ͼ2װ�õ��ŵ���:
���ܸ��õ������ж�����SO2,��ֹ����Ⱦ����;
������
(2)������e��������Ҫ����:һ���ڷ�Ӧ������,�ܲ���Һ����,����Һ�⡱������ֹSO2�����ݳ�����Ⱦ����;��������
(3)��˵����SO2���������ʵ������������
(4)��Ӧһ��ʱ���,�õι���ȡA�Թ��е���Һ��������ˮ��Ϊ����,�����������������ӵijɷ����������ֿ���:
��:ֻ����Fe3+;��:ֻ����Fe2+;��:����Fe3+����Fe2+��
Ϊ��֤��Ŀ�����,ѡ�������Լ�,��д���пո�:
| A��ϡHCl��Һ | B��ϡH2SO4��Һ | C��KSCN��Һ | D��KMnO4��Һ |
 Fe(SCN)3��
Fe(SCN)3�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ⶨ�������Ȼ��Ƶ�С�մ��̬��Ʒ��NaHCO3�����������ɲ����������ַ�����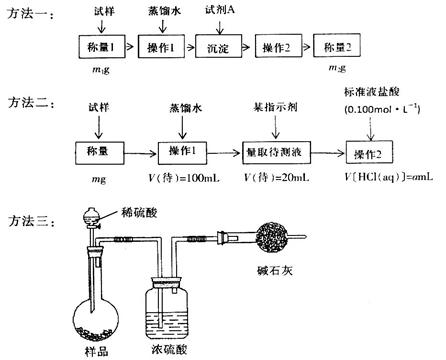
�����ģ���ʹ�û�ѧ�Լ���ʹ��ʵ���ҳ���������
��Ҫ��ش��������⣺
��1������һ�ǽ�HCO3-����ת��Ϊ���������أ����Լ�AΪ______________���ѧʽ����Һ������2����_______________________��
��2������������1��Ҫ�õ��IJ����������ձ���������������Ҫ___________________������2��������__________������Ʒ��NaHCO3����������Ϊ_________���ú�m��a�ı���ʽ��ʾ����
��3�����ݷ����������õ�ʵ��װ�ã����˳������������⣬����ⶨ��ʵ��������_______________����ϸ������ʵ��װ�ã��ɴ˲�õ����ݼ������ʵ�����п���ƫ��Ҳ�п���ƫ�ͣ�ƫ�ߵ�ԭ�������______________________________��ƫ�͵�ԭ�������____________________________��
��4�������ĵ�ʵ��ԭ����________________���û�ѧ����ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ȸʯ����Ҫ�ɷ�ΪCu2(OH)2CO3���������������������������������ʵ
�����Կ�ȸʯΪԭ���Ʊ�CuSO4��5H2O���������ͼ��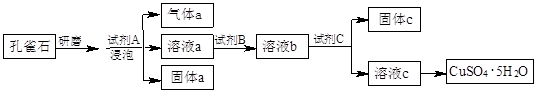
��1����ĥ��ȸʯ��Ŀ���� �����ݿ�ȸʯ���Լ�A��ѡ�ù�����ϡ���ᣬ�����a�� (�ѧʽ)��
��2���Լ�B��Ŀ���ǽ���Һ�е�Fe2+ת��ΪFe3+�����Լ�B��ѡ�� ������ţ���
A������KMnO4��Һ B��˫��ˮ C��Ũ���� D����ˮ
��Ӧ�����ӷ���ʽΪ�� ��
��3���Լ�C���ڵ�����ҺpH��ʹFe3+ת��Ϊ�������Է��롣���Լ�C��ѡ�� ������ţ���
A��ϡ���� B��NaOH��Һ C����ˮ D��CuO
����C�Ļ�ѧʽΪ ��
��4��1 mol����ͨ�����ȵ�Cu2(OH)2 CO3���Բ���1.5 mol����ͭ����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��������Fe(OH)3��Ksp=1��10-39����Ҫ����Һ�е�Fe3+ת��ΪFe(OH)3������ʹ��Һ��c(Fe3+)������1��10��3 mol��L�����轫��Һ������pH= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ�ⶨNa2CO3��NaHCO3����������Na2CO3��������������ȡһ����������Ʒ����ͬѧ����ͼI��ʾװ�ò�������CO2���������ͬѧ����ͼII��ʾװ��ͨ������ܵ����ز�������CO2����������֪����ϡ�����������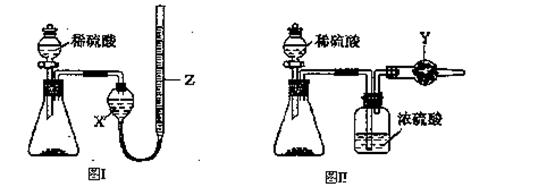
��l��ʢ��ϡ�������������Ϊ ��
��2���Լ�XΪ ���Լ�YΪ ��
��3����ͬѧ�ڽ���ʵ��ʱ��Ϊ��С��Ӧע��������У���ѡ����ĸ�� ��
| A������ǰӦʹ����װ����ȴ������ |
| B������Z�ĸ߶�ʹ����װ������Һ����ƽ |
| C������ʱ������Z�ڰ�Һ����͵����� |
| D������ǰӦͨ��һ������N2ʹ���ɵ�CO2ȫ����������װ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
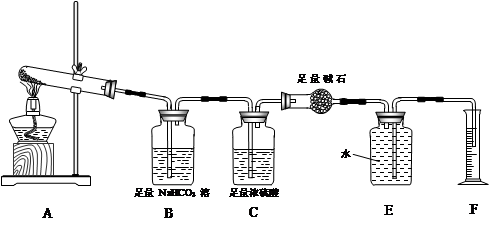
FeCl3���ִ���ҵ������Ӧ�ù㷺��ij��ѧ�о���ѧϰС��ģ�ҵ�����Ʊ���ˮFeCl3�����ø���ƷFeCl3��Һ�����ж���H2S��
I�����������ϵ�֪����ˮFeCl3�ڿ������׳��⣬����������������������Ʊ���ˮ FeCl3��ʵ�鷽����װ��ʾ��ͼ�����ȼ��г�װ����ȥ���������������£�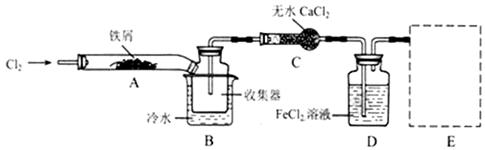
�ټ���װ�õ������ԣ�������������
��ͨ������Cl2���Ͼ�װ���еĿ�����
���þƾ�������м�·���������Ӧ��ɣ�
�ܡ���
����ϵ��ȴ��ֹͣͨ��Cl2�����ø����H2�Ͼ�Cl2�����ռ����ܷ⡣
��ش��������⣺
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ�������������������������������������� ��
��2�����������У�Ϊ��ֹFeCl3��������ȡ�Ĵ�ʩ�У������ţ��������������� ��
��3�����û��װ��C����ƣ��ᵼ��
��4�������ӷ���ʽ��ʾ���߿�E��������װ�ú��Լ������ã� ��
��5����װ��D�еĸ���ƷFeCl3��Һ����H2S���õ���������д����Ӧ�����ӷ���ʽ���������� ��
��Ӧ�������ռ��������ù�����ȫ����ϡ���ᣬС��ͬѧ��������Һ���������ӵijɷ������ֹ۵㣺��ֻ��Fe3+����ֻ��Fe2+������ �� ��������
Ϊ̽����Һ����ɣ�ʵ�����£�
| ʵ�鲽�� | ʵ������ | ʵ����ۼ���Ӧ���ӷ���ʽ |
| ��ȡ����������Һ���Թ��У���������KSCN��Һ�� | _________________�� | ˵��������ڲ�����������ٻ�۳�������Ӧ�����ӷ���ʽ��_____________�� |
| ����ȡ����������Һ���Թ��У������������� KMnO4��Һ�� | ��Һ�Ϻ�ɫ��ȥ | ˵����________________________�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ʯ����ɺ��������ƣ��������Al2O3��������Fe2O3�����ʣ�������ȡ�طʺ�ұ�������� Ҫԭ�ϣ��䲽�����£�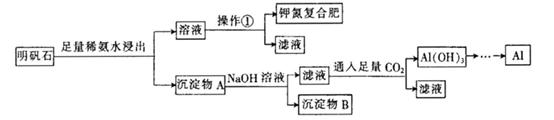
�ش��������⣺
(1) ����ʯ����������ϡ��ˮ�н���ʱ������Ӧ�����ӷ���ʽΪ________________
(2) ������A�г�����Fe2O3�⣬������ ________��________��
(3) �����ٵ�����Ϊ________����ȴ�ᾧ�����ˡ�
(4) ��14mol . L-1��Ũ��ˮ����480 mL 2 mol . L?1ϡ��ˮ��
�����õ�����ƿ�����________��
��ϡ��Ũ��ˮ�õ��ձ��Ͳ��������ϴ�ӣ�________________________
�����в����������Ƶ�ϡ��ˮŨ�ȵ�Ӱ��(�ƫ����ƫС������Ӱ�족
a��ϴ�Ӻ������ƿ������������ˮ��________��
b��Ũ��ˮ��ȡ�����õ���Ͳ������ˮϴ��2?3�Σ�����ϴ��Һת������ƿ��: ��
(5) ȷ���ص����Ϸ��к��м�Ԫ�صķ��� �� ��
(6) Ϊ�ⶨ�ص����Ϸ��е�Ԫ�ص�������������ȡmg�ص����Ϸʣ�����������NaOHŨ��Һ���ȣ�ʹ����������ȫ���ݳ����ռ����İ�������ɱ�״���µ����ΪV mL����ص����Ϸ��е�Ԫ�ص���������Ϊ________ (�ú�m��V�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com