
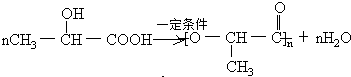
��֪��������������Ĵ������£������������ᣬ�����ʽ��C3H6O3��
(1)д��������ת��Ϊ����Ļ�ѧ����ʽ��__________________________________________��
(2)��ɫ����������Һ��ʹ��ɫʯ����Һ���ɫ���ܹ��ڼ��ȡ�Ũ���������������������������������Ӧ����
�������к��еĹ����ŵķ��ź������ǣ�____________________________��
���������л�����һ����(��CH3)��������Ľṹ��ʽΪ___________________________��
(3)д�����л�ѧ����ʽ��
����������ķ�Ӧ��____________________________________________________________��
�������������������Ӧ��______________________________________________________��
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)д��������ת��Ϊ����Ļ�ѧ����ʽ��__________________________________________��
(2)��ɫ����������Һ��ʹ��ɫʯ����Һ���ɫ���ܹ��ڼ��ȡ�Ũ���������������������������������Ӧ����
�������к��еĹ����ŵķ��ź������ǣ�____________________________��
���������л�����һ����(��CH3)��������Ľṹ��ʽΪ___________________________��
(3)д�����л�ѧ����ʽ��
����������ķ�Ӧ��________________________________________________��
�������������������Ӧ��____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��д��������ת��Ϊ����Ļ�ѧ����ʽ��________________________________________��
��2��ȡ
��3���Ҵ���ͭ�������Ĵ������£����Է�����Ӧ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��������ͭ�������Ĵ������£����Է������Ƶķ�Ӧ���Ҳ���Ϊ
2CH3CHO+2H2O��������ͭ�������Ĵ������£����Է������Ƶķ�Ӧ���Ҳ���Ϊ![]() �����ƶ�����Ľṹ��ʽ��_____________��
�����ƶ�����Ľṹ��ʽ��_____________��
��4��д����ѧ����ʽ��
����������ķ�Ӧ��__________________________________________________��
�������������������Ӧ��______________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com