
A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʡ�����֮�������µķ�Ӧ��ϵ��
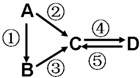
(1)��B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ��B��C��һ�������·�Ӧ���ɵ�A�Ǵ�������Ҫ�ɷ֣�д���÷�Ӧ�Ļ�ѧ����ʽ��__________________________________��
(2)��D���ʾ������ԣ��ڡ��۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧ��ͨ�������һ����������ЧӦ����Ҫ���塣�жϵ���A��Ԫ�������ڱ��е�λ�ã�__________________��
(3)��A��̫���ܵ���õĹ�����ϡ�C��DΪ���Σ������������ơ������Ԫ��Ϊͬһ���壬����Һ���Լ��ԡ�д���ڷ�Ӧ�Ļ�ѧ����ʽ��__________________________________��
(4)��A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A���ڡ��ݷ�Ӧ���õ�ͬһ�ַǽ������ʡ�C����Һ����ʴ��ӡˢͭ��·�壬д���÷�Ӧ�����ӷ���ʽ��__________________________��
(5)��AΪ����ɫ���壬C��D���������C������������Ҫ���ʣ� B��C�ɷ�Ӧ����A����д��B�ĵ���ʽ______________��
 һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д� Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ����յ�һ����Ҫ������ͭϴ����Ŀ������ͭҺ[���������ͭ��1������ˮ]���������������в�����CO��CO2�����壬ͭҺ����CO�ķ�Ӧ�Ƿ��ȷ�Ӧ���䷴Ӧ����ʽΪ��Cu(NH3)2Ac��CO��NH3 [Cu(NH3)3CO]Ac��Ac��ʾ�������
[Cu(NH3)3CO]Ac��Ac��ʾ�������
���������գ�
��1�����Ҫ���������Ӧ�ķ�Ӧ���ʣ����Բ�ȡ�Ĵ�ʩ�� ����ѡ���ţ�
a. ��ѹ b. ����NH3��Ũ�� c.���� d.��ʱ���߲���
��2��ͭҺ�еİ������ն�����̼��д���÷�Ӧ�Ļ�ѧ����ʽ��
��3������ͭҺ����CO��ͭҺ�����IJ������裨ע�����պ���������������
.
��4��ͭҺ�����Ԫ���У�������Ԫ��ԭ�Ӱ뾶�Ӵ�С������˳��Ϊ
ͨ���Ƚ� ���жϵ���������Ԫ�صķǽ�����ǿ����
��5����֪CS2��CO2���ӽṹ���ƣ�CS2�ĵ���ʽ��
CS2�۵����CO2����ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������һ����������Ѫ�ܼ�����ҩ����ɻ�����E��M��һ�������ºϳɵõ������ַ�Ӧ�����ԣ�
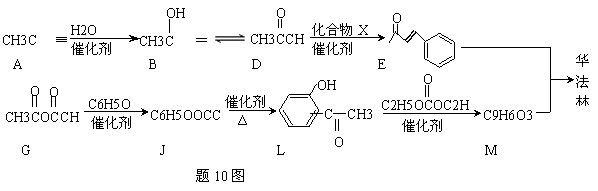

��A������Ϊ ��A B�ķ�Ӧ����Ϊ
B�ķ�Ӧ����Ϊ
��D E�ķ�Ӧ�У�����Ļ�����X������Cu(OH)2��Ӧ����ש��ɫ�����Ļ�ѧ����ʽΪ
E�ķ�Ӧ�У�����Ļ�����X������Cu(OH)2��Ӧ����ש��ɫ�����Ļ�ѧ����ʽΪ
��G JΪȡ����Ӧ������һ��������еĹ�������
JΪȡ����Ӧ������һ��������еĹ�������
��L��ͬ���칹��Q�Ƿ����ᣬQ
 R(C8H7O2Cl)
R(C8H7O2Cl)
 S
S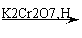
 T��T�ĺ˴Ź�������ֻ������壬Q�Ľṹ��ʽΪ
T��T�ĺ˴Ź�������ֻ������壬Q�Ľṹ��ʽΪ  ��R
��R S�Ļ�ѧ����ʽΪ
S�Ļ�ѧ����ʽΪ
���� 10ͼ�У������ϳ����߷��ӻ�����ķ������
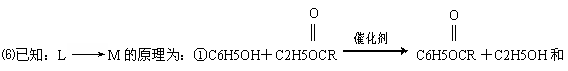

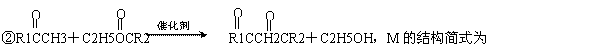

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���г��ӷ�����ȷ����(����)
| ѡ�� | ���ᴿ������ | ���� | �����Լ� | ���ӷ��� |
| A | CO2(g) | SO2(g) | ����NaHSO4��Һ��ŨH2SO4 | ϴ�� |
| B | NH4Cl(aq) | Fe3��(aq) | NaOH��Һ | ���� |
| C | Cl2(g) | HCl(g) | ����NaHCO3��Һ��ŨH2SO4 | ϴ�� |
| D | Na2CO3(s) | NaHCO3(s) | �� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�ʵ������ͽ��ͻ���۶���ȷ��ѡ����(�� ��)
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ��SO2ͨ����ɫʯ����Һ�� | ��ɫ��ȥ | SO2����Ư���� |
| B | ��Ũ����ε�pH��ֽ�� | ��ֽ��� | Ũ����������� |
| C | ������NO2���ܱղ������������ˮ�� | ����ɫ��dz | ��Ӧ2NO2 N2O4�Ħ�H<0 |
| D | ��AgCl�����еμ�KI��Һ | ��ɫ���� ��Ϊ��ɫ | �ܶȻ�:AgI�� AgCl��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ������������ԭ��Ӧ����
A��п����ϡ������ B��������طֽ�
C��ʵ�����Ʊ����� D��̼��ˮ�����ڸ����·�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��д��ȷ����
A��NaHSO4��Һ��Ba��OH��2��Һ��Ӧ�����ԣ�H++SO42-+Ba2++OH��=BaSO4��+H2O
B��NaClO��Һ��FeCl2��Һ��ϣ�Fe2++2ClO��+2H2O =Fe��OH��2��+2HC1O
C��NH4HSO3��Һ������NaOH��Һ��Ӧ��NH+4+OH��=NH3��+H2O
D����1mol/LNaAlO2��Һ��1.5mol/L��HC1��Һ��������Ȼ�ϣ�
6AlO��2+9H++3H2O=5Al(OH)3��+Al3+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C��D��E���ֳ���������������±��е������γɵģ�
| ������ | K+ Na+ Cu2+ Al3+ |
| ������ | SO42- HCO3- NO3- OH- |
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���˵�C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ��ֻ��B��CΪ��ɫ(����ɫ�겣��)��
���ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬA�зų���ɫ���壬C��D�в�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɣ�
��������ʵ�����
(1)д��B��D�Ļ�ѧʽ��B ��D ��
(2)����1 mol A����Һ�뺬1 mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ļ�ѧʽΪ ��
(3)��A��Һ�м�����������ʯ��ˮ�������ӷ���ʽΪ ��
(4)C��������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ��������ͼ��ʾ��TԪ�ص��������������۵Ĵ�����Ϊ0�������ж���ȷ����
| R | ||
| T | Q | W | |
A��ԭ�Ӱ뾶�Ĵ�С��W��Q��R
B����̬�⻯����ȶ��ԣ�R��Q��T
C����Ӧ�����������ǿ����W��Q��T
D��R�ֱ���T��Q��W�γɻ�����ľ����Ϊ���Ӿ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com