
����Ŀ�����±���������������ѡ���ʵ�������������ֵ����(ÿ������ֻ��ѡ��һ��)���ö��Ե缫��ÿ�ֵ������Һ���е�⡣�ش��������⣺
������ | H+��Na+��Ag+ |
������ | Cl-��SO42-��NO3- |
��1���������ų������������ų��������ҵ�����Һ��pH��С������ѡ�õĵ���ʵĻ�ѧʽ��___________�������ĵ缫��ӦʽΪ___________��
��2�����������������������ų�����������ѡ�õĵ���ʵĻ�ѧʽ��___________�������ĵ缫��ӦʽΪ_______________________________________________________________��
��3������ͼ��ʾװ�õ������ֵ����M�ı�����Һ��д���õ����з�����Ӧ���ܻ�ѧ����ʽ��____________________________��
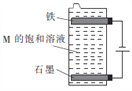
���𰸡� H2SO4 2H++2e-=H2�� AgNO3 4OH--4e-=O2��+2H2O(��2H2O--4e-=O2��+4H+) NaCl+H2O![]() NaClO+H2��
NaClO+H2��
��������������������⿼���ö��Ե缫���������Һ�Ĺ��ɣ��缫��Ӧʽ�͵���ܷ���ʽ����д������AgCl������ˮ��Ag2SO4����ˮ��Ag+ֻ����NO3-�γɵ������Һ��
��1�������ӷŵ�˳��ΪAg+![]() H+
H+![]() Na+�������ӵķŵ�˳��ΪCl-
Na+�������ӵķŵ�˳��ΪCl-![]() OH-
OH-![]() ����������������ų�H2�������ų�O2�������ϵ��ˮ���������Һ������ΪAgNO3��Һ���������Һ�в�������Cl-������ÿ������ֻ��ѡ��һ�Σ��������Һ����ΪH2SO4��Na2SO4��������Һ��pH��С�ĵ���ʵĻ�ѧʽΪH2SO4�������ĵ缫��ӦʽΪ2H++2e-=H2����
����������������ų�H2�������ų�O2�������ϵ��ˮ���������Һ������ΪAgNO3��Һ���������Һ�в�������Cl-������ÿ������ֻ��ѡ��һ�Σ��������Һ����ΪH2SO4��Na2SO4��������Һ��pH��С�ĵ���ʵĻ�ѧʽΪH2SO4�������ĵ缫��ӦʽΪ2H++2e-=H2����
��2�����������������������ų�O2����ѡ�����Ϊ�����ý����ĺ������Σ�����ѡ����ʵĻ�ѧʽΪAgNO3������ΪOH-�ŵ磬�����缫��ӦʽΪ4OH--4e-=O2��+2H2O��
��3������ÿ������ֻ��ѡ��һ�Σ�������ֵ������ҺΪNaCl��Һ���ڵ�����FeΪ�������缫��ӦʽΪ2H2O+2e-=H2��+2OH-��ʯīΪ�����������缫��ӦʽΪ2Cl--2e-=Cl2������ⷴӦΪ2NaCl+2H2O![]() 2NaOH+H2��+Cl2�����������ɵ�Cl2���������ɵ�NaOH������ӦCl2+2NaOH=NaCl+NaClO+H2O��������Ӧ��ӵõ����з�����Ӧ���ܻ�ѧ����ʽΪNaCl+H2O
2NaOH+H2��+Cl2�����������ɵ�Cl2���������ɵ�NaOH������ӦCl2+2NaOH=NaCl+NaClO+H2O��������Ӧ��ӵõ����з�����Ӧ���ܻ�ѧ����ʽΪNaCl+H2O![]() NaClO+H2����
NaClO+H2����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ���仯�����ڹ�ũҵ�������ճ�������Ӧ�÷dz��㷺��
(1) �ɻ�ͭ����ȡͭ�ķ�Ӧ����Ϊ��
2Cu2S(s)��3O2(g)===2Cu2O(s)��2SO2(g)����H����768.2 kJ��mol��1��
2Cu2O(s)��Cu2S(s)===6Cu(s)��SO2(g)����H����116.0 kJ��mol��1��
���Ȼ�ѧ����ʽ��Cu2S(s)��O2(g)===2Cu(s)��SO2(g)����H�� ________kJ��mol��1��
�ڻ�õĴ�ͭ���⾫��������ʱ��ͭ��________����
(2) �⻯��ͭ��һ�ֺ�ɫ���壬�������з�Ӧ�Ʊ���4CuSO4��3H3PO2��6H2O===4CuH����4H2SO4��3H3PO4��
�ٸ÷�Ӧ��ԭ����____________(д��ѧʽ)��
�ڸ÷�Ӧÿת��3 mol���ӣ�����CuH�����ʵ���Ϊ________��
(3) �Ȼ�ͭ��Һ��ͭ�����ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��c(Cl��)�Ĺ�ϵ��ͼ��ʾ��
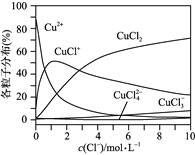
�ٵ�c(Cl��)��9 mol��L��1ʱ����Һ����Ҫ��3�ֺ�ͭ���ֵ�Ũ�ȴ�С��ϵΪ__________________________��
����c(Cl��)��1 mol��L��1���Ȼ�ͭ��Һ�У�����AgNO3��Һ����ͭ���ּ�ת�������ӷ���ʽΪ___________________________��___________________________(��д����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л�����ϩ��������ϩ�õ������ļ��飬�����ν���ͨ�����������Լ���ϴ��ƿ
A.����ʯ��ˮ��ŨH2SO4B.��ˮ��NaOH��Һ��ŨH2SO4
C.���Ը��������Һ��ŨH2SO4D.ŨH2SO4�����Ը��������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ����ܲ����������ǣ� ��
A.��AlCl3��Һ�мӹ�����NaOH��Һ
B.��NaAlO2��Һ�мӹ���������
C.��AlCl3��Һ��ͨ�������CO2
D.��NaAlO2��Һ��ͨ�������CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������25mL0.1mol��L-1MOH��Һ����μ���0.2mol��L-1HA��Һ���к͵ζ�������ͼ��ʾ(����仯���Բ���)���ش��������⣺
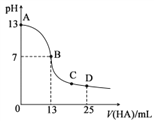
��1��д��MOH��ˮ��Һ�еĵ��뷽��ʽ��___________��
��2��HA�ĵ���ƽ�ⳣ��Ϊ___________ mol��L-l(д��������)��
��3��D��ʱ����Һ��c(A-)+c(HA)___________ 2c(M+)(����>����<������=��)����Һ������Ũ�ȴ�С˳��Ϊ__________������ʱ��û����Һ��pH=a����c(HA)+c(H+)=___________mol��L-l(�ú���ĸa�ļ������ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���̶�������ܱ������У�����4molA��2molB������Ӧ��2A��g��+B��g��![]() 3C��g��+D��g���ﵽƽ��ʱ��C��Ũ��ΪWmol/L����ά������������¶Ȳ��䣬���������ַ����ı���ʼ���ʣ��ﵽƽ���C��Ũ����ΪWmol/L���ǣ�������
3C��g��+D��g���ﵽƽ��ʱ��C��Ũ��ΪWmol/L����ά������������¶Ȳ��䣬���������ַ����ı���ʼ���ʣ��ﵽƽ���C��Ũ����ΪWmol/L���ǣ�������
A. 4molA+2molB
B. 2molA+1molB+3molC+1molD
C. 3molC+1molD+1molB
D. 3molC+1molD
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��ݷ�Ӧԭ����Ƶ�Ӧ�ã�����ȷ����(����)
A. CO32����H2O![]() HCO3����OH������Al2(SO4)3��Һ���������
HCO3����OH������Al2(SO4)3��Һ���������
B. Al3����3H2O![]() Al(OH)3��3H����������ˮ
Al(OH)3��3H����������ˮ
C. TiCl4��(x��2)H2O(����) ![]() TiO2��xH2O����4HCl���Ʊ�TiO2����
TiO2��xH2O����4HCl���Ʊ�TiO2����
D. SnCl2��H2O![]() Sn(OH)Cl����HCl�������Ȼ�������Һʱ����Ũ����
Sn(OH)Cl����HCl�������Ȼ�������Һʱ����Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����14 g Na2O��Na2O2�Ļ�������������ˮ�г�ַ�Ӧ�����ɱ�״���µ�����1.12 L��������Һ�����Ϊ400 mL���Լ��㣺
��1��ԭ�������Na2O������Ϊ________ g��
��2��������Һ�����ʵ���Ũ��Ϊ________ mol��L��1��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com