
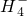 ��Cl-��C
��Cl-��C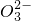 ��S
��S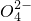 ���ֱַ�ȡ100mL�����ȷ���Һ��������ʵ�飺
���ֱַ�ȡ100mL�����ȷ���Һ��������ʵ�飺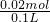 =0.2 mol/L��
=0.2 mol/L��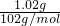 =0.01mol����Һ��Al3+�����ʵ���Ũ��=
=0.01mol����Һ��Al3+�����ʵ���Ũ��=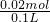 =0.2 mol/L��
=0.2 mol/L��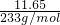 =0.05mol��SO42- ���ʵ���Ũ��=
=0.05mol��SO42- ���ʵ���Ũ��= =0.5 mol/L���ʴ�Ϊ��NH4+��0.2 mol/L��Al3+��0.2 mol/L��SO42-��0.5 mol/L��
=0.5 mol/L���ʴ�Ϊ��NH4+��0.2 mol/L��Al3+��0.2 mol/L��SO42-��0.5 mol/L��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| X |
| X |
| H2O |
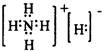
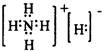
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | A | B | C |
| ��ʼŨ��/mol?L-1 | 0.020 | 0.020 | 0 |
| ƽ��Ũ��/mol?L-1 | 0.016 | 0.016 | 0.0080 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͼ�����Ͽ�Ϊ�����ṩ�ܶ���Ϣ��������ij����ͼ�����ϵ�ʹ�ã����в���ȷ���ǣ� ��
A�����ݽ����˳������жϽ����ܷ��û���ϡ�����е���
B�������۵����ݱ����жϽ����¶�ʱ�����ֹ��������ˮ��Һ����������������
C������Ԫ�����ڱ����ж�ͬ��������Ԫ�ص����������ö��Ե缫�����ˮ��Һʱ�������ȷ�����Ӧ
D����������ε��ܽ��Ա����ж�ijЩ���ֽⷴӦ�ܷ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com