
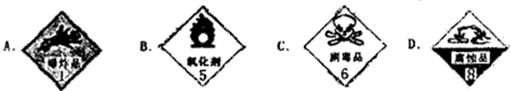
��ͼ�������Լ�ƿ��ǩ�ϵ����ݣ�

(1)����������ʵ���Ũ����________mol/L��
(2)ij��ѧ��ȤС������������ʵ�ʵ��̽��ʱ����Ҫ240 mL��4.6 mol/L��ϡH2SO4������Ҫȡ________mL�ĸ����ᣬ�������������ϡ�͵�ʵ�����Ϊ________��
(3)������4.6 mol/LϡH2SO4�Ĺ����У����������������Һ���ʵ���Ũ���к�Ӱ��(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
��δ����ȴ������Һע������ƿ�У�________��
������ƿ��1 mol/LϡH2SO4��ϴ��________��
�۶���ʱ���ӹ۲�Һ��________��
(4)�����£�ijѧ��ȡ�������Լ�ƿ�е�������һ�ྻ�Թ��У����뼸Ƭ���������Ƭ����û�����ݲ�����Ҳδ������Ƭ�ܽ⣬������ͬѧ������ԭ��________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

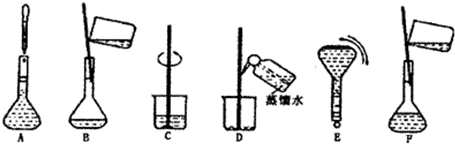
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

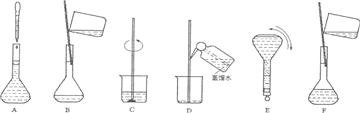
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���

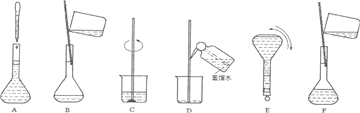
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com