
 ��ѧ��Ӧ������������ѧ��Ӧ���п����̶ȵ���������������ijͬѧ�ⶨ��ѧ��Ӧ���ʲ�̽����Ӱ�����ص�ʵ�飮
��ѧ��Ӧ������������ѧ��Ӧ���п����̶ȵ���������������ijͬѧ�ⶨ��ѧ��Ӧ���ʲ�̽����Ӱ�����ص�ʵ�飮| ʵ����� | ���V/mL | ʱ��/s | |||
| Na2S2O3��Һ | ������Һ | ��ˮ | ˮ | ||
| �� | 10.0 | 2.0 | 4.0 | 0.0 | t1 |
| �� | 8.0 | 2.0 | 4.0 | 2.0 | t2 |
| �� | 6.0 | 2.0 | 4.0 | Vx | t3 |
���� ��1����ʵ��װ�ÿ�֪����ʵ����ͨ��������һ��ʱ��������ռ����������������ⶨ��Ӧ���ʣ��ݴ˷���ʵ����Ʒ��
��2��SO2������ˮ����������õ�SO2�����ƫС��
��3�����ݷ�ӦS2O32-+2H+�TH2O+S��+SO2����֪������ͨ���ⶨ���ɵĵ������������ʵʱ�ⶨ��Һ��������Ũ�������H+�����ʵ����ĸı������Ӷ������Ӧ���ʣ�
��4��Ϊ��̽����Ӧ��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬�����Na2S2O3��Һ��Ũ�Ȳ�ͬ�⣬Ӧ��������Ӱ������һ�£�����������ʵ����Na2S2O3��Һ��Ũ�Ȣ٣��ڣ��ۣ��ݴ˷�����ѧ��Ӧ���ʺͷ�Ӧ����ʱ��t�Ĵ�С��
��� �⣺��1����ʵ��װ�ÿ�֪����ʵ����ͨ��������һ��ʱ��������ռ����������������ⶨ��Ӧ���ʣ��ʻ�ȱ�ٵ������������
�ʴ�Ϊ�������
��2��SO2������ˮ����������õ�SO2�����ƫС����ݴ˼�����ġ�n��H+���͡�C��H+���Լ�V��H+�����С��
�ʴ�Ϊ��SO2�Ჿ������ˮ��
��3�����ݷ�ӦS2O32-++2H+�TH2O+S��+SO2����֪������ͨ���ⶨһ��ʱ��������ɵĵ������������ʵʱ�ⶨ��Һ��������Ũ�������H+�����ʵ����ĸı������Ӷ�����V=$\frac{\frac{��n��{H}^{+}��}{V}}{��t}$�����㣬
�ʴ�Ϊ���ⶨһ��ʱ�����������������������ʵʱ�ⶨ��Һ��������Ũ�ȣ���
��4��Ϊ��̽����Ӧ��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬�����Na2S2O3��Һ��Ũ�Ȳ�ͬ�⣬Ӧ��������Ӱ������һ�£���Ӧʹ��Һ�����Ϊ16mL����Vx=4mL������������ʵ����Na2S2O3��Һ������٣��ڣ��ۣ�����Ϻ���Һ�����ͬ���ʻ�Ϻ�Na2S2O3Ũ�Ȣ٣��ڣ��ۣ���֪��ѧ��Ӧ���ʢ٣��ڣ��ۣ���Ӧ����ʱ��t�Ĵ�Сt1��t2��t3��
�ʴ�Ϊ��4��t1��t2��t3��
���� ������Ҫ����ѧ����ʵ��̽�����������ʱ��ע��ʵ���ԭ������Ϥʹ�öԱ�ʵ���п��Ʊ�������Ҫ�㣬�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
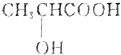 ����һ�������칹�壬��Ϊ������к���һ������̼ԭ�ӣ�
����һ�������칹�壬��Ϊ������к���һ������̼ԭ�ӣ�| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��Na+����c��CH3COO-����c��OH-����c��H+�� | B�� | c��Na+����c��CH3COO-����c��H+����c��OH-�� | ||
| C�� | c��Na+����c��CH3COO-����c��H+��=c��OH-�� | D�� | c��Na+��=c��CH3COO-����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��Ba2+����c��OH-����c��Na+����c��CO32-�� | B�� | c��OH-����c��Na+����c��Ba2+����c��CO32-�� | ||
| C�� | c��OH-����c��Ba2+����c��Na+����c��CO32-�� | D�� | c��Na+����c��OH-����c��Ba2+����c��CO32-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʵ���Ϊ1mol | B�� | ��������64g | ||
| C�� | ��Sԭ����Ŀ����NA | D�� | ��Oԭ�ӵ����ʵ���С��2mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| KMnO4������Һ ��Ũ��/mol•L-1 | ��Һ��ɫ����ʱ�� t/min | ||
| ��1�� | ��2�� | ��3�� | |
| 0.01 | 14 | 13 | 12 |
| 0.001 | 6 | 7 | 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
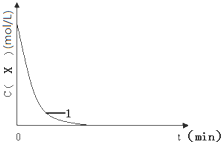 ���½��У���
���½��У���| ��� | ���ձ��м�����Լ�����������mL�� | ���� | ��ʼ����ʱ�䣨min�� | ||||
| 0.1 mol•L��1 KI��Һ | H2O | 0.01 mol•L��1 X ��Һ | 0.1 mol•L��1 ˫��ˮ | 1 mol•L��1 ϡ���� | |||
| 1 | 20.0 | 10.0 | 10.0 | 20.0 | 20.0 | �� | 1.4 |
| 2 | 20.0 | m | 10.0 | 10.0 | n | �� | 2.8 |
| 3 | 10.0 | 20.0 | 10.0 | 20.0 | 20.0 | �� | 2.8 |
| 4 | 20.0 | 10.0 | 10.0 | 20.0 | 20.0 | 5��Fe2��SO4��3 | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ӵ糡�������£����巢����Ӿ����˵�������Ǵ���ɵ� | |
| B�� | ˮ�ķе������ķе�ߣ�����Ϊˮ�������γ������ | |
| C�� | �������������Ӿ�һ���������� | |
| D�� | �е��ʲμӻ����ɵķ�Ӧһ����������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Է���������ͬ�����Ԫ��Ҳ��ͬ�Ļ�����һ����ͬ���칹�� | |
| B�� | ���Ƿ���������һ�������ɸ�CH2ԭ���ŵ����ʣ��˴�һ����ͬϵ�� | |
| C�� | �������ʵ����Ԫ����ͬ����Ԫ�ص���������Ҳ��ͬ��������һ����ͬ���칹�� | |
| D�� | ����ʽ��ͬ�IJ�ͬ�л���һ����Ϊͬ���칹�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com