
(11��)�±������������г��������ʣ������г������ǵģ���Ҫ���ɷ֡�
|
��� |
�� |
�� |
�� |
�� |
�� |
�� |
�� |
|
���� |
�ƾ� |
���� |
��� |
ʳ�� |
ͭ���� |
�������� |
�մ� |
|
��Ҫ �ɷ� |
CH3CH2OH |
CH3COOH |
NaOH |
NaCl |
Cu |
SO2 |
Na2CO3 |
��1������Ա��Т١��ߵ���Ҫ�ɷֽ��з��ࣨ���ţ���
�����ε��� �����ڵ���ʵ��� �����ڷǵ���ʵ��� ��
��2��д�������ڵ�ˮ��Һ��߷�Ӧ�Ļ�ѧ����ʽ ��
����������߷�Ӧ�����ӷ���ʽ ��
��ijͬѧ�âݺ�Ũ���Ṳ�����Ʊ��ޣ���ѧ����ʽΪ��
Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
��������ת�Ƶķ������Ŀ��������ת��0.1molʱ�����Ļ�ԭ��������Ϊ g��
�� �ܢ�(1��)���ڢۢܢ�(1��);�٢�(1��)
�� 2CH3COOH+Na2CO3=2CH3COONa+CO2��+H2O (2��); H++CO32-=HCO3- (2��)
�� �������ת�Ƶķ������Ŀ��1�֣�
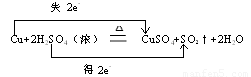
3.2g(2��)
��������
�����������1�������ε���ʳ�Ρ��մ����ڵ���ʵ��д��ᡢ��ʳ�Ρ��մ����ڷǵ���ʵ��оƾ�������������
��2��������̼���Ʒ�Ӧ�ķ���ʽΪ��2CH3COOH+Na2CO3=2CH3COONa+CO2��+H2O������������̼���Ʒ�Ӧ�����ӷ���ʽΪ��H++CO32-=HCO3-����дʱ��Ӧ�ر�ע��������Զ��ٶԷ�Ӧ��Ӱ�졣
��3��������ԭ��Ӧ�е���ת������ı�ʾ����˫���ű�ʾ����ͼ��
���Ļ�ԭ����������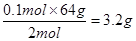
���㣺����ʣ����ӷ���ʽ����д��������ԭ��Ӧ
�����������ۺϿ����֪ʶ��϶࣬�����ǻ����Ե����ݣ����ڻ����⡣���������͵Ŀ��飬���ɿ���ѧ���Ի���֪ʶ�����գ��ֿ��Խ�϶�����ݡ��Ի���֪ʶ��������������˿����������ĵ÷������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ�ϰ�һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
(11��)�±������������г��������ʣ������г������ǵģ���Ҫ���ɷ֡�
| ��� | �� | �� | �� | �� | �� | �� | �� |
| ���� | �ƾ� | ���� | ��� | ʳ�� | ͭ���� | �������� | �մ� |
| ��Ҫ �ɷ� | CH3CH2OH | CH3COOH | NaOH | NaCl | Cu | SO2 | Na2CO3 |
 CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(11��) (1)��A��B��C���־��壬�ֱ���C��H��Na��Cl����Ԫ���е�һ�ֻ����γɣ��������־������ʵ�飬������±���
|
��Ŀ |
�۵�/��[��Դ:Z��xx��k.Com] |
Ӳ�� |
ˮ���� |
������ |
ˮ��Һ��Ag����Ӧ |
|
A |
811 |
�ϴ� |
���� |
ˮ��Һ(������)���� |
��ɫ���� |
|
B |
3 500 |
�ܴ� |
���� |
������ |
����Ӧ |
|
C |
��114.2 |
��С |
���� |
Һ̬������ |
��ɫ���� |
�� ����Ļ�ѧʽ���������ͷֱ�Ϊ��
A________ ____��B_______ ___��C____ ____��
�� ���������Ӽ�����÷ֱ�Ϊ��
A______ ___��B______ __��C_______ _��
��2��ˮ������֮Դ���������ǵ�����������ء��ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮҲ��һ�ֳ��õ��Լ���
��д����H2O���ӻ�Ϊ�ȵ��������__________����һ�ּ��ɣ���
��ˮ�������ض����������õ�һ��H�����γ�ˮ�������ӣ�H3O���������ж��������̵��������������ǣ� ��
A����ԭ�ӵ��ӻ����ͷ����˸ı� B��������״�����˸ı�
C�����Ļ�ѧ���ʷ����˸ı� D�����еļ��Ƿ����˸ı�
���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ͼ(δ��˳������)������ľ���������ͬ����______(������Ӧ�ı����д)

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(11��)(1)��A��B��C���־��壬�ֱ���C��H��Na��Cl����Ԫ���е�һ�ֻ����γɣ��������־������ʵ�飬������±���
| ��Ŀ | �۵�/�� | Ӳ�� | ˮ���� | ������ | ˮ��Һ��Ag����Ӧ |
| A | 811 | �ϴ� | ���� | ˮ��Һ(������)���� | ��ɫ���� |
| B | 3 500 | �ܴ� | ���� | ������ | ����Ӧ |
| C | ��114.2 | ��С | ���� | Һ̬������ | ��ɫ���� |
�� ����Ļ�ѧʽ���������ͷֱ�Ϊ��
A________ ____��B_______ ___��C____ ____��
�� ���������Ӽ�����÷ֱ�Ϊ��
A______ ___��B______ __��C_______ _��
��2��ˮ������֮Դ���������ǵ�����������ء��ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮҲ��һ�ֳ��õ��Լ���
��д����H2O���ӻ�Ϊ�ȵ��������__________����һ�ּ��ɣ���
��ˮ�������ض����������õ�һ��H�����γ�ˮ�������ӣ�H3O���������ж��������̵��������������ǣ� ��
A����ԭ�ӵ��ӻ����ͷ����˸ı� B��������״�����˸ı�
C�����Ļ�ѧ���ʷ����˸ı� D�����еļ��Ƿ����˸ı�
���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ͼ(δ��˳������)������ľ���������ͬ����______(������Ӧ�ı����д)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com