
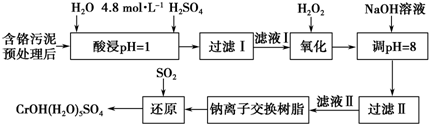
| ������ | Fe3+ | Mg2+ | Al3+ | Cr3+ |
| ��ʼ����ʱ��pH | 2.7 | - | - | - |
| ������ȫʱ��pH | 3.7 | 11.1 | 8 | 9����9�ܽ⣩ |
���� ��1��������Һ���ƵIJ�����̷������õ�����������Ҫ����Ӧ��������Һ������ƿ�Ͷ�����Ҫ�Ľ�ͷ�ιܣ�����һ�����ʵ���Ũ�ȵ���Һ����IJ��������У��ձ����������������ܡ�����ƿ�ͽ�ͷ�ιܣ���Ũ��������ΪxmL������$\frac{1.84x��98%}{98}$=0.250L��4.8mol•L-1���㣻
��2�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ���ӳ���ȡʱ�䡢�ӿ��ܽ��ٶȵȴ�ʩ�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+��������ܽ�����Ϊ����߽�ȡ�ʣ����������¶����������ܽ�ȣ�����Ӵ��������Ӧ���ʣ���ӿ�����ٶȵȣ�
��3������˫��ˮ�����ʷ�����˫��ˮ��ǿ�����ԣ���������ԭ�Ե����ʣ�����������ԭ��Ӧ�����غ㡢ԭ���غ���ƽ��д���ӷ���ʽ��
��4�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+�����������������������ΪCr2O72-������NaOH��Һʹ��Һ�ʼ��ԣ�Cr2O72-ת��ΪCrO42-����ҺPH=8��Fe3+��Al3+������ȫ����Һ������������ҪNa+��Ca2+��Mg2+������PH=8��������������������������ܽ���ǿ����Һ��Ӱ������ӵĻ������ã�
��5�������ӽ�����֬���� �������Ǹ����Ӻ�þ���ӣ�
��6����������ͼ�е�ת����ϵ�Ͳ����϶�������Ļ�ԭ�ԣ�����������ԭ��Ӧԭ��������д��
��� �⣺��1������һ�����ʵ���Ũ�ȵ���Һ����IJ��������У��ձ����������������ܡ�����ƿ�ͽ�ͷ�ιܣ���Ũ��������ΪxmL������$\frac{1.84x��98%}{98}$=0.250L��4.8mol•L-1��֪��x=65.2mL��
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�65.2��
��2�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�ǣ��ӳ���ȡʱ�䡢�ӿ��ܽ��ٶȵȴ�ʩ��
�ʴ�Ϊ�����߷�Ӧ�¶ȣ������������ı������
��3��˫��ˮ��ǿ�����ԣ���������ԭ�Ե����ʣ�Cr3+�л�ԭ�ԣ�Cr3+�ܱ�˫��ˮ����Ϊ�����ӣ��Ա������������ӷ��룬
�ʴ�Ϊ��2Cr3++3H2O2+H2O=Cr2O72-+8H+��
��4�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+�����������������������ΪCr2O72-������NaOH��Һʹ��Һ�ʼ��ԣ�Cr2O72-ת��ΪCrO42-����ҺPH=8��Fe3+��Al3+������ȫ����Һ������������ҪNa+��Ca2+��Mg2+������PH=8��������������������������ܽ���ǿ����Һ��Ӱ������ӵĻ������ã�
�ʴ�Ϊ��Na+��Mg2+��Ca2+��pH����8��ʹ����Al��OH��3�ܽ�����AlO2-������Ӱ��Cr��III�������������ã�
��5�������ӽ�����֬�����������Ǹ����Ӻ�þ���ӣ��ʴ�Ϊ��Ca2+��Mg2+��
��6������������л�ԭ�ԣ�����Һ����ͨ�����ӽ��������Һ��Na2CrO4����Ϊ���ᣬNa2CrO4������ԭΪCrOH��H2O��5SO4��ˮ��Һ����������������Һ�����ᷴӦ���������ƣ�����ԭ���غ������д��ƽ��3SO2+2Na2CrO4+12H2O=2CrOH��H2O��5SO4��+Na2SO4+2NaOH��
�ʴ�Ϊ��3SO2+2Na2CrO4+12H2O=2CrOH��H2O��5SO4��+Na2SO4+2NaOH��
���� ���⿼�������ӷ���ʽ����ѧ����ʽ����д�����ʵķ����֪ʶ�㣬�ѶȽϴ�ע���������Һ��PHֵ����Һ�е����ӽ��з��룬���ӵ�ԭ���ǣ���ȥ�����Ҳ������µ����ʣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{18}{{N}_{A}}$ | B�� | $\frac{18}{{N}_{A}}$g•mol-1 | C�� | 18NA g | D�� | $\frac{18}{{N}_{A}}$g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaHSO4?Na++H++SO42- | B�� | HCO3-+H2O�TH3O++CO32- | ||
| C�� | HClO�TH++ClO- | D�� | H2S?H++HS- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʵ���ұ����ʯʱӦע���ܷ⣬�������������ﴦ | |
| B�� | ������Ӧ����1molC2H2�����ڳ��³�ѹ�����Ϊ2.24L | |
| C�� | ��ʯ��ˮ��Ӧ����������ԭ��Ӧ | |
| D�� | ����C2H2������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�Һ���ɷ���ֲ���ͺ�ˮ | |
| B�� | �ù��˷��ɳ�ȥʳ���л��е�Fe��OH��3���� | |
| C�� | �þƾ���ȡ��ˮ�еĵ� | |
| D�� | ������ɳ�ȥMg�л��е�Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ۢڢ� | B�� | �٢ۢܢ� | C�� | �ڢ٢ۢ� | D�� | �ۢ٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
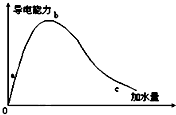 ��һ���¶��£��������ˮϡ�͵Ĺ����У���Һ�ĵ���������ͼ��ʾ����ش�
��һ���¶��£��������ˮϡ�͵Ĺ����У���Һ�ĵ���������ͼ��ʾ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �����ᣨHCN�� | ̼�ᣨH2CO3�� | ����ᣨHF�� |
| K=6.2��10-10 | Ka1=4.2��10-7 | K=6.61��10-4 |
| A�� | ��������Һ��ͨ��CO2��2F-+H2O+CO2�T2HF+CO32- | |
| B�� | NaCN��HCN�Ļ����Һ�У�2c��Na+���Tc��CN-��+c��HCN�� | |
| C�� | 0.2 mol•L-1 HCN��Һ��0.1mol•L-1NaOH��Һ�������Ϻ���Һ��pH��7 | |
| D�� | 25��ʱͬŨ�ȵ�NaCN��NaF��Һ��pHֵǰ��С�ں��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com