
��������Һ�м��� NaCl��Һ���ް�ɫ�������������� KI��Һ�л�ɫ����������˵��������Һ�� �������ӷ��ţ�����Ũ�Ⱥ�С�����������������������ɫ������д���˷�Ӧ�����ӷ���ʽ
����һ����ɫ���壬������A��(NH4)2SO4��B��Al2(SO4)3��C��NaCl��D��AgNO3��E��KOH��F��KI��G��BaCl2�е�һ�ֻ�����ɣ��ð�ɫ��������ˮ�ý�ϡ�������Һ������Һ��ʹ��̪�ʺ�ɫ�����ڸ���Һ�м�����ϡ���ᣬ�а�ɫ�������ɡ�
�ش��������⣨��д��Ӧ�Լ��Ĵ��룩��
�Ÿð�ɫ������һ�������ڵĻ������� ��
�Ƹð�ɫ���������ٴ����ļ��ֻ������д�����ܵ������
һ������� ���� ��
��һ������� ��
 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
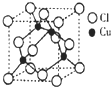 ��2013?���϶�ģ������ͭ�ĵ����Խ����������������ڵ�����ҵ��
��2013?���϶�ģ������ͭ�ĵ����Խ����������������ڵ�����ҵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
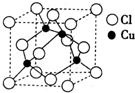 ��2012?�Ͳ�һģ������ͭ�ĵ����Խ����������������ڵ�����ҵ��
��2012?�Ͳ�һģ������ͭ�ĵ����Խ����������������ڵ�����ҵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и���4�¹�����ѵ�����ۻ�ѧ�Ծ��������棩 ���ͣ�������
����ͭ�ĵ����Խ����������������ڵ�����ҵ��
��1��д��ͭ�Ļ�̬ԭ�Ӽ۵��ӵ����Ų�ʽ________��
��2��ͭ��ij���Ȼ��ᄃ��ľ����ṹ����ͼ��ʾ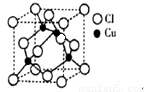 �����Ȼ���Ļ�ѧʽΪ__________��
�����Ȼ���Ļ�ѧʽΪ__________��
��3�����Ȼ�ͭ��Һ�м������Ũ��ˮ��Ȼ����������Ҵ�����Һ����������ɫ��[Cu(NH3)4]Cl2���塣��������Nԭ�ӵ��ӻ���ʽΪ__________����������ɫ�����к��еĻ�ѧ������ͨ���ۼ��⣬����________��_____________��
��4��NH3�ķе��pH3����ԭ����________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���Ĵ�ʡ������ʮ�����¿������ۣ���ѧ���� ���ͣ�ѡ����
����ʵ���ܴﵽԤ��Ŀ�ĵ���
|
��� |
ʵ �� �� �� |
ʵ �� Ŀ �� |
|
A |
���ѻ������м������Ը��������Һ������ɫ��ȥ |
˵�������к��мױ��ȱ���ͬϵ�� |
|
B |
��CH3CH2Br��NaOH��Һ��ϼ��ȣ��ٵμ�AgNO3��Һ��δ����dz��ɫ���� |
CH3CH2Brδ����ˮ�� |
|
C |
pH��Ϊ1�����ᡢ������Һ�ֱ�������ˮϡ��m���� n�����pH��ͬ |
m��n |
|
D |
������Һ�м�������ϡ���ᡢ���ȣ�Ȼ���������Һ�ټ��� |
��֤������ǿ�����������Ƿ���ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡģ���� ���ͣ������
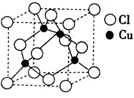
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com