
����Ŀ����.�����ϣ���ʦ������μ���Ũ������ϡ���ᣬijѧϰС����Ʒ�������
���� | ���� | |
�� | ������Ͷ���� | �����ΪŨ���� |
�� | ������Ƭ | �����̼�����ζ��ΪŨ���� |
�� | ����������ʢˮ��С�ձ��� | ������ΪŨ���� |
�� | �ò�����պŨ��ˮ�������ƿ�� | ð������ΪŨ���� |
�� | ����μӵ����������� | �����ΪŨ���� |
(1)���Ϸ����У����е���______________������ţ�
(2)����һ�������Ľ����ܳ�Ϊ���з�����________________���Ľ�����Ϊ____________��
(3)��ȫ�������__________����Ϊ_________________________________________��
��.ʵ������Ũ��������1.0mol/L������Һ480mL���ش��������⣺
(1)����ͼ��ʾ��������������Һ�϶�����Ҫ����____________������ĸ��������������Һ����Ҫ�õ��IJ���������_________________________�����������ƣ���
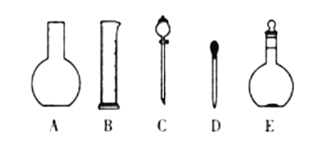
(2)����ƿ�ϱ�������5���е�_____________������ţ�
��ѹǿ ���¶� ������ ��Ũ�� �ݿ̶���
(3)�����ƹ����У����в���ʹ������ҺŨ��ƫ�����______________������ţ���
��δ��ϡ�ͺ��H2SO4��Һ��ȴ�����¾�ת�Ƶ�����ƿ��
�ڶ���ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ���̶���
�۶���ʱ�����ӿ̶���
��ʹ������ƿǰ������ˮϴ����û����
(4)����ʵ��������������Һ������Ͳȡ��������Ϊ98%���ܶ�Ϊ1.84g/mL��Ũ��������Ϊ_____________________mL��������С�����һλ��
���𰸡��٢ۢ� �� ������ų�����ʹ��Ƭ�ܽ����ϡ���� �� �������ѻӷ����ᣨ���߸߷е��� �� AC �ձ��������� �ڢۢ� �� 36.8
��������
��.����ϡ�����Ũ�������ʲ�ͬ���ʵ�飻
��. (1)��������һ�����ʵ���Ũ����Һ�õ�������ѡ����������Һ�����ѡ������ƿ�Ĺ��
(2)��������ƿ������
(3) �������������ʵ����ʵ�������Һ�����Ӱ�죬����C= ![]() ������������
������������
(4)����c= ![]() ����Ũ��������ʵ���Ũ�ȣ�������Һϡ�����ʵ����ʵ������������ҪŨ���������
����Ũ��������ʵ���Ũ�ȣ�������Һϡ�����ʵ����ʵ������������ҪŨ���������
(1)Ũ���������ˮ�ԣ���ʹ���ڣ�ϡ����û����ˮ�ԣ��ʢٿ��У�Al��ϡ���ᷴӦ����������Ũ�����Al�����ۻ����ʢڲ����У�Ũ�����ϡ����ϡ�����ж��ų��ȣ���Ũ����ų�������ԶԶ����ϡ���ᣬ�ʢۿ��У�Ũ�����ϡ���ᶼû�лӷ��ԣ������ò�����պŨ��ˮ����ʢ�����ƿ�ڲ��������̣��ʢܲ����У�Ũ���������ˮ�ԣ�ϡ����û����ˮ�ԣ�����Ũ���ᡢϡ����ֱ�ӵ����������ϣ������Ũ���ᣬ�ʢݿ��У�
���е��Ǣ٢ۢݣ�
(2) ����һ�������Ľ����ܳ�Ϊ���з����Ǣڣ��������ܺ�ϡ���ᷴӦ��������������Ũ����ۻ������Ե���һ�£�������Ƭ��������ų�����ʹ��Ƭ�ܽ����ϡ���
(3)��Ũ����߷е㣬���ӷ������Դ��Լ�ƿ��������ð���̣��ʢ���ȫ����
��. (1)����һ�����ʵ���Ũ����Һ�õ������У�������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ�����Ҫ����������ƿ�ͷ�Һ©����Ҫ����450mL��ҺӦѡ��500mL����ƿ�����Ի�ȱ�ٵ��������ձ������������ʴ�Ϊ��AC���ձ�����������
(2)����ƿ�ϱ��У��¶ȡ��������̶��ߣ���ѡ���٢ۢݣ�
(3)��δ��ϡ�ͺ��H2SO4��Һ��ȴ�����¾�ת�Ƶ�����ƿ�У���ȴ����Һ���ƫС�����ʵ����ʵ���Ũ��ƫ�ߣ���ѡ��
�ڶ���ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ���̶��ߣ�������Һ���ƫ�����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ʲ�ѡ��
�۶���ʱ�����ӿ̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ��ʲ�ѡ��
��ʹ������ƿǰ������ˮϴ����û�����ʵ����û��Ӱ�죬�ʲ�ѡ��
��ȷ���Ǣ٣�
(4)��������Ϊ98%���ܶ�Ϊ1.84g/mL��Ũ��������ʵ���Ũ��c=![]() =18.4mol/L������ҪŨ�������ΪV������1.0mol/L������Һ480mL��Ҫ��500mL����ƿ����������Һϡ�����ʵ����ʵ����������ã�18.4mol/L��V=1mol/L��500mL�����V=36.8mL��
=18.4mol/L������ҪŨ�������ΪV������1.0mol/L������Һ480mL��Ҫ��500mL����ƿ����������Һϡ�����ʵ����ʵ����������ã�18.4mol/L��V=1mol/L��500mL�����V=36.8mL��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ��ȡŨ�Ⱦ�Ϊ0.1molL-1�Ĵ�����Һ�Ͱ�ˮ��Һ��20mL���ֱ���0.1molL-1NaOH��Һ��0.1molL-1��������к͵ζ����ζ�������pH��μ���Һ������仯��ϵ��ͼ��ʾ������˵����ȷ����
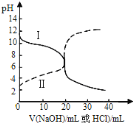
A.���ߢμ���Һ��10mLʱ![]()
B.���ߢμ���Һ��20mLʱ��![]()
C.���ݵζ�����,�ɵ�![]()
D.�����ߢ��֪,ѡ�÷�̪��ѡ�ü�����ָʾ��������NaOH��Һ�������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����������η���E��ֱ���η���G��Ӧ�������������η���L��ֱ���η���M(���E���ӵ�Ԫ�ص�ԭ��������С��10�����G���ӵ�Ԫ��Ϊ��������Ԫ��)�������ж�����ȷ����(����)
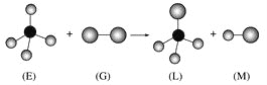
A. ���³�ѹ�£�L��һ��Һ̬�л���
B. E�Ķ������ֻ�����ֽṹ
C. G����ǿ�����Ժ�Ư����
D. ������Ӧ��������ȡ����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
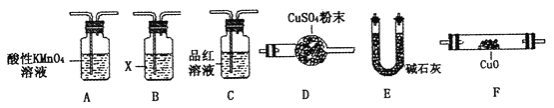
����Ŀ����ҵ�ϳ�����������ʢװ��Ũ���ᡣijѧϰС��Ϊ�о����ʲ�������Ũ����ķ�Ӧ�����������̽�����
��ȡ������̼�ظ֣�6.0 g����15.0 mLŨ�����У����ȣ����Ӧ��õ���ҺM���ռ�������N��
��1����С��ͨ�������������ΪM��Һ�мȺ���Fe3����Ҳ���ܺ���Fe2������ȷ����Һ������Fe2����Ӧ������Լ���___________��ѡ����ţ���
a��KSCN��Һ����ˮ b�����ۺ�KSCN��Һ
c��NaOH��Һ d������KMnO4��Һ
��2��ȡ320 mL����״��������Nͨ��������ˮ�У�Ȼ���������BaCl2��Һ������____________ ��______________����������ƣ�������õ�����2.33 g������N����ˮ��Ӧ�Ļ�ѧ����ʽΪ_______________��������֪����N��SO2���������Ϊ_______________��
��3����С��ͨ���������ijɷֺ�SO2��������ķ�������Ϊ����N�л����ܺ���H2������һ������Q������ΪQӦ����_____________��������________________���û�ѧ����ʽ��ʾ����
��4��ȡһ����N�����������������һ����ͬʱ����N�к���H2������Q��װ�ã��г��������ܺͼ���װ��ʡ�ԣ�������������˳����_____________������ĸ��������A��������_____________��B���Լ�X�Ļ�ѧʽ��_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һ�к���H����Mg2����Al3�����������ӣ���μ���0.1mol/L NaOH��Һ������NaOH��Һ��������ɳ���֮��Ĺ�ϵ����ͼ��ʾ��������˵����ȷ����

A.������0��50mL ʱ��������Ӧֻ�У�Mg2��+2OH��= Mg(OH)2��Al3��+3OH��= Al (OH)3��
B.B�����ɳ���������Ϊ13.6g
C.����C�����Һ�м���0.1mol/L ����100 mL ��������ȫ���ܽ�
D.H����Mg2����Al3���������������ʵ���Ũ��֮��Ϊ2��2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2�ֳ���Ԫ����ɵĻ�����G���й�ת����ʵ����Ϣ���£�
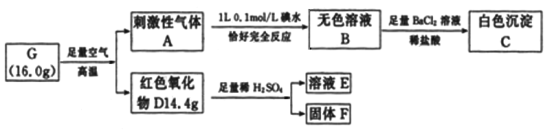
��ش��������⣺
(1)G��______________���ѧʽ����
(2)д��A��B�����ӷ���ʽ_____________________________________________��
(3)��DΪ�����F�Ǻ�ɫ�������ʣ�д��D��ϡ���ᷴӦ�����ӷ���ʽ_____________��
(4)C���������Ϊ________________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ�ת���ڸ�����������ʵ�ֵ���
A. Na![]() Na2O
Na2O![]() Na2CO3
Na2CO3
B. Al![]() Al2O3
Al2O3![]() Al(OH)3
Al(OH)3
C. Fe(OH)2![]() Fe(OH)3
Fe(OH)3![]() Fe2O3
Fe2O3
D. Na2CO3(aq)![]() NaHCO3
NaHCO3![]() CO2
CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ը�����(��FeO��Cr2O3��Al2O3��SiO2��)Ϊԭ���Ʊ���������ص�ʵ�鲽����ͼ��
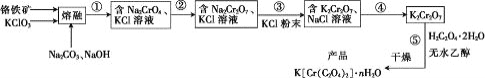
�ش��������⣺
(1)����������װ����ͼ������W�IJ��ʿ�����________(�����������մ�������������)��FeO��Cr2O3��KClO3��Na2CO3������Ӧ������Fe2O3��KCl��Na2CrO4��CO2�Ļ�ѧ����ʽΪ_______________��
(2)���ں�Ĺ����к�Na2CrO4��Fe2O3��Na2SiO3��NaAlO2��KCl�ȣ�����ٵľ��岽��Ϊˮ�������ˣ���pHΪ7~8��������а�Сʱ�����ȹ��ˡ���һ�ι��������е���Ҫ�ɷ�Ϊ________������pHΪ7~8��������а�Сʱ����Ŀ����__________��
(3)�����������ᣬ�����ϡ����ʱ������Ӧ�����ӷ���ʽΪ________��
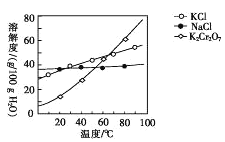
(4)����ܰ����ľ��������____��������õ�K2Cr2O7���塣(�й����ʵ��ܽ��������ͼ��ʾ)
(5)������������ֹ��������м���һ��ˮ�������ƾ���ĥ�����õĹ�������������������________��
(6)�������ط������ⶨK[Cr(C2O4)2]��nH2O��Ʒ�����ᾧˮ��Ŀ������Ʒ���ȵ�80��ʱ��ʧ��ȫ���ᾧˮ��ʧ��16.8%��K[Cr(C2O4)2]��nH2O������n=____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ѧ֪ʶ,�ش���������:
��1����12.5g CuSO4��5H2O����ˮ���γ�1L��Һ�����ʵ����ʵ���Ũ��Ϊ_________mol��L��1
��2����ԭ�ӵ����ʵ��������������HCl��H2��NH3��CH4����ͬ��ͬѹ�£��������������֮��V(HCl)��V(H2)��V(NH3)��V(CH4)=______________��
��3������100mL 1.00 mol��L��1H2SO4��Һ����Ҫ����Ͳ��ȡŨ���ᣨ�ܶ�Ϊ1.84g��cm��3��������������Ϊ98%�������Ϊ_____________mL��
��4����Ӧ2K2S+ K2SO3+3H2SO4=3S��+3 K2SO4+3H2O�У��������뻹ԭ�������ʵ���֮��Ϊ______������Ӧ����0.6molS����Ӧ��ת�Ƶĵ���Ϊ____________mol��
��5����������5�����ʣ���CO2 ��Mg ������ ��NaCl ��Na2CO3�������������ڵ���ʵ���_____________������ţ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com