��2009��3���𣬴�ī���硢�����ȹ�����ɢ��ȫ����ļ���H1N1�����б������飬����ȫ���ע�ͻ���Ӧ�ԣ�����ר�ұ�ʾ���������������������ǿ��������������Ԥ������H1N1�����У�
��1����̼���� ����ѧʽ2Na
2CO
3?3H
2O
2�� �׳ƹ���˫��ˮ����ɫ�ᾧ��������̼��������ˮ������ʱ���ֽ�����̼���ƺ������⣬�ǺܺõĹ������ͷż���
������д����������ĵ���ʽ
��������Ԫ�صĻ��ϼ���
-1
-1
��
��H
2O
2��ʱ����Ϊ��ҵ�������������С���ɫ�������������ƣ��������ɿ�ҵ��Һ�е��軯���KCN���������·�Ӧʵ�֣�
KCN+H
2O
2+H
2O=A+NH
3������������A�Ļ�ѧʽΪ
KHCO3
KHCO3
��H
2O
2����Ϊ����ɫ����������������
�ڷ�Ӧ��ϵ�в��������ʣ���Ի�������Ⱦ��
�ڷ�Ӧ��ϵ�в��������ʣ���Ի�������Ⱦ��
��
��ijǿ���Է�Ӧ��ϵ�У���Ӧ��������ﹲ�������ʣ�O
2��MnO
4-��H
2O��Mn
2+��H
2O
2��H
+����֪�÷�Ӧ��H
2O
2ֻ���������¹��̣�H
2O
2��O
2��д���÷�Ӧ�����ӷ���ʽ
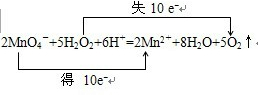
2MnO4-+5H2O2+6H+�T2Mn2++5O2��+8H2O
2MnO4-+5H2O2+6H+�T2Mn2++5O2��+8H2O
��
��ʵ������O
2��Ҫ��4�ַ������û�ѧ����ʽд������һ��
��
��2��̼����ˮ��Һ�Լ��ԣ������ӷ���ʽ������ԭ��
CO32-+H2O?HCO3-+OH-
CO32-+H2O?HCO3-+OH-
��pHֵ��ͬ�Ģ�̼������Һ���ڴ�������Һ��������������Һ����Ũ���ɴ�С��˳���ǣ�
�ڣ��٣���
�ڣ��٣���
����д��ţ���
��3��Ư���������� ��NaClO
2�� �ڳ��ºڰ����ɱ���һ�꣮������ȶ��ɷֽ⣬��Ӧ�����ӷ���ʽΪ��
HClO
2��ClO
2��+H
++Cl
-+H
2O��δ��ƽ�����ڸ÷�Ӧ�У�����1molClO
2����ʱ��ת�Ƶĵ�������
NA
NA
����
��4��ClO
2���ȶ�������NaOH��Һ��H
2O
2��Ӧ��ת��Ϊ���ȶ����������� ��NaClO
2�����÷�Ӧ�Ļ�ѧ����ʽΪ
2ClO2+2NaOH+H2O2�T2NaClO2+O2��+2H2O
2ClO2+2NaOH+H2O2�T2NaClO2+O2��+2H2O
��




 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�


 �������������������ǿ�������������ɷ�����H1N1���У�
�������������������ǿ�������������ɷ�����H1N1���У�