

 Ϊ���������ı�ʶ��
Ϊ���������ı�ʶ�� Ϊ���������ı�ʶ����ѡ��c��
Ϊ���������ı�ʶ����ѡ��c��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
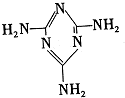 ���׳ƣ������������������з�Ӧ�ϳɣ�
���׳ƣ������������������з�Ӧ�ϳɣ�
| ||
| ||
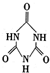 ��������֮����ͨ��
��������֮����ͨ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ȵμ���ˮ���ٵμ�KSCN��Һ���Ժ�ɫ |
| B���ȵ�KSCN��Һ�����Ժ�ɫ���ٵμ���ˮ���Ժ�ɫ |
| C���μ�NaOH��Һ���Ȳ�����ɫ������������ɫ�����ʺ��ɫ�� |
| D��ֻ��Ҫ�μ�KSCN��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ʹ��������δ��������������� |
| B���ϳ���ά���ά�����л��߷��ӻ����� |
| C���⻯ѧ�������γ�������β���еĵ��������й� |
| D������ʹ�÷����Ͻ�֣��úϽ��۵㡢Ӳ�Ⱥ�ǿ�Ⱦ��ȴ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
. |
| v |
A��
| ||
B��
| ||
C��
| ||
D��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������̹�����2��������ԭ��Ӧ |
| B�����������ӵ�Ũ�ȶ������˱仯 |
| C����Һ����ɫ�����˱仯 |
| D����Ӧ�����м������������Ҳ�г������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NH4+��NO3-��OH- |
| B��Ca2+��OH-��HCO3- |
| C��Na+��S2-��Cl- |
| D��Fe3+��Na+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2KOH+H2SO4=K2SO4+2H2O |
| B��2H2O2=2H2O+O2�� |
| C��CO2+2NaOH=NaCO3+H2O |
| D��NH3+HCl=NH4Cl |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com