
����Ŀ���л���K��һ������ȱѪ������ҩ���ϳ�·�����£�
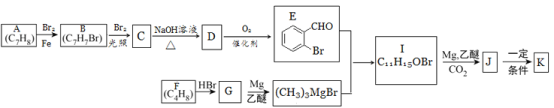
��֪����.�л���K��һ�����������г����������1����Ԫ����
��.R��Br ![]() RMgBr
RMgBr 
�ش��������⣺
(1)�л���B������Ϊ________��D�ĺ��������ŵ�������______��
(2)F��G�ķ�Ӧ����Ϊ_____��
(3)J�Ľṹ��ʽΪ______��K�ķ���ʽΪ_____��
(4)E�������Һ��Ӧ�Ļ�ѧ����ʽΪ______��
(5)��֪B��һ�������¿���ˮ������X����X��Ϊͬ���칹���Һ��б������л�����_____��(����X����)��д�����к˴Ź���������4�����շ壬�ҷ�ֵ��Ϊ3��2��2��1�Ľṹ��ʽ____(��дһ��)��
(6)����������Ϣ��ѧ֪ʶ��д���Լ���ͱ���ȩΪԭ�ϣ��ϳɱ���ϩ��·������ͼ(�����Լ���ѡ)____��
���𰸡�����ױ���2-��ױ� �ǻ� �ӳɷ�Ӧ ![]() C12H14O2
C12H14O2 ![]() +2Ag(NH3)2OH
+2Ag(NH3)2OH ![]()
![]() +2Ag��+3NH3+H2O 4
+2Ag��+3NH3+H2O 4 ![]() ��
��![]() CH4
CH4![]() CH3Br
CH3Br![]() CH3MgBr��
CH3MgBr�� ![]()
![]()
![]()
![]()
![]()
��������
��A-E�Ŀ�ͼ���ƿ�֪��DΪ![]() ��CΪ
��CΪ![]() ��BΪ
��BΪ![]() ����F-G�ͷ�Ӧ������֪��FΪCH2=C(CH3)2��HBr�ӳ�����G��CH3C(CH3)2Br����������֪�ͷ�Ӧ����֪I�Ľṹ��ʽΪ
����F-G�ͷ�Ӧ������֪��FΪCH2=C(CH3)2��HBr�ӳ�����G��CH3C(CH3)2Br����������֪�ͷ�Ӧ����֪I�Ľṹ��ʽΪ![]() ��J�Ľṹ��ʽΪ
��J�Ľṹ��ʽΪ![]() ��K�Ľṹ��ʽΪ
��K�Ľṹ��ʽΪ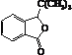 ��
��
��1�����л���B�Ľṹ��ʽ![]() ����֪B������Ϊ����ױ���D�Ľṹ��ʽ��
����֪B������Ϊ����ױ���D�Ľṹ��ʽ��![]() �����������ŵ��������ǻ����ʱ����Ϊ������ױ���2-��ױ����ǻ���
�����������ŵ��������ǻ����ʱ����Ϊ������ױ���2-��ױ����ǻ���
��2�����ݷ�Ӧ������F��G�Ľṹ��֪��FΪCH2=C(CH3)2��HBr�ӳ�����G��CH3C(CH3)2Br��������F��G�ķ�Ӧ����Ϊ�ӳɷ�Ӧ���ʱ����Ϊ���ӳɷ�Ӧ��
��3�����ݷ���֪/span>J�Ľṹ��ʽΪ![]() ��K�ķ���ʽΪC12H14O2���ʱ����Ϊ��
��K�ķ���ʽΪC12H14O2���ʱ����Ϊ��![]() ��C12H14O2��
��C12H14O2��
��4���ɷ���֪EΪ![]() ����������Һ��Ӧ�Ļ�ѧ����ʽΪ��
����������Һ��Ӧ�Ļ�ѧ����ʽΪ��![]() +2Ag(NH3)2OH
+2Ag(NH3)2OH ![]()
![]() +2Ag��+3NH3+H2O���ʱ����Ϊ��
+2Ag��+3NH3+H2O���ʱ����Ϊ��![]() +2Ag(NH3)2OH
+2Ag(NH3)2OH ![]()
![]() +2Ag��+3NH3+H2O��
+2Ag��+3NH3+H2O��
��5���л���B(�ṹ��ʽΪ![]() )��һ��������ˮ��IJ���XΪ
)��һ��������ˮ��IJ���XΪ![]() �����以Ϊͬ���칹���Һ��б������л����У�
�����以Ϊͬ���칹���Һ��б������л����У�![]() ��
��![]() ��
��![]() ��
��![]() ����4�֣����к˴Ź���������4�����շ壬�ҷ�ֵ��Ϊ3��2��2��1�Ľṹ��ʽΪ
����4�֣����к˴Ź���������4�����շ壬�ҷ�ֵ��Ϊ3��2��2��1�Ľṹ��ʽΪ![]() ��
��![]() ���ʱ����Ϊ��4��
���ʱ����Ϊ��4��![]() ��
��![]() ��
��
��6���Լ���ͻ�����A��![]() ��Ϊԭ�ϣ��ϳɱ���ϩ��·������ͼ��CH4
��Ϊԭ�ϣ��ϳɱ���ϩ��·������ͼ��CH4![]() CH3Br
CH3Br![]() CH3MgBr���ɱ���ȩ��
CH3MgBr���ɱ���ȩ��![]() ��Ϊԭ�ϣ�
��Ϊԭ�ϣ�![]()
![]()
![]()
![]()
![]() ���ʱ����Ϊ��CH4
���ʱ����Ϊ��CH4![]() CH3Br
CH3Br![]() CH3MgBr��
CH3MgBr�� ![]()
![]()
![]()
![]()
![]() ��
��


 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ�к���80����Ԫ�أ�����Ҫ��������Դ���⣬ͬʱ��ˮ����ǿ�����Ȼ����������Ϊ���������Ⱦ�����ṩ�˹����Ŀռ䡣
��1������֪��ͬpH�����£�ˮ��Һ��̼Ԫ�صĴ�����̬����ͼ��ʾ������˵������ȷ����______________������ĸ��ţ���

a��pH=8ʱ����Һ�к�̼Ԫ�ص�����Ҫ��HCO3-
b��A�㣬��Һ��H2CO3��HCO3-Ũ����ͬ
c����c(HCO3-)=c(CO32-)ʱ��c(H+)>c(OH -)
��������pH=8.4��ˮ��Һ�м���NaOH��Һʱ������Ӧ�����ӷ���ʽ��______________��
(2)��ˮpH�ȶ���7.9��8.4֮�䣬�������̵�����CO2��SO2�����ռ���
�ٺ�ˮ�к��е�OH -���������̵����е�CO2ͬʱΪ��ˮ�Ѹƣ�����CaCO3��д���˷�Ӧ�����ӷ���ʽ��__________��
����֪��25��ʱ��H2CO3����ƽ�ⳣ��K1=4.3��10-7 K2=5.6��10 -11
H2SO3����ƽ�ⳣ��K1=1.5��10-2 K2 =6.0��10-8
��ˮ�к��е�HCO3-����������SO2���ù��̵����ӷ���ʽ��_______________��
(3)ϴ��������ĺ�ˮ�����ԣ��账���������ŷš������ʺ�ˮ���ͬʱ������������ų�����CO2����һ�ִ�������Ч��ʽ��
��ͨ��O2�ɽ����Ժ�ˮ�е���(IV)�������÷�Ӧ�����ӷ���ʽ��_______________��
��������ʽʹ������ˮpH���ߵ�ԭ����_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���0.10mol/L CH3 COOH��Һ�ζ�10.00mLŨ�Ⱦ�Ϊ0.10 mol/L NaOH��NH3��H2O�Ļ��Һ�������Һ����Ե����������������ı仯��������ͼ��ʾ����֪��Ka(CH3COOH)=1. 8��10-5��Kb( NH3��H2O) =1. 8�� l0-5�����������������
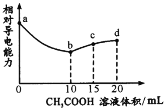
A.ab��ΪCH3COOH��NaOH���к���
B.b��ˮ�ĵ���̶����
C.c��3c(Na+)=2c(CH3 COOH)+2c(CH3 COO-)
D.d��c(Na+)��c(NH![]() )��c(OH-)��c(H+)
)��c(OH-)��c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽����������Է�Ӧ:mA(g) +nB(g)![]() cZ(g) ��H��Ӱ�죬��A��B���ʵ���֮��Ϊ m��n��ʼ��Ӧ��ͨ��ʵ��õ���ͬ�����´ﵽƽ��ʱZ�����ʵ���������ʵ������ͼ��ʾ�������ж���ȷ���ǣ� ��
cZ(g) ��H��Ӱ�죬��A��B���ʵ���֮��Ϊ m��n��ʼ��Ӧ��ͨ��ʵ��õ���ͬ�����´ﵽƽ��ʱZ�����ʵ���������ʵ������ͼ��ʾ�������ж���ȷ���ǣ� ��
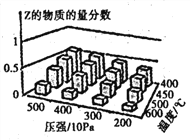
A.�ں��º��������£����Ѵﵽƽ�����ϵ�м�������Z��ƽ�������ƶ�,Z�ĺ�������
B.�����¶ȣ������淴Ӧ���ʶ�����ƽ�ⳣ������
C.��H��0 ����m + n ��c
D.��H��0 ����m +n��c
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��ȷ����
A.Ǧ�����س��ʱ��������Ӧ��PbSO4+2H2O+2e-��PbO2+4H++![]()
B.�ö��Ե缫����Ȼ�ͭ��Һ2Cl-+2H2O![]() Cl2��+H2��+2OH-
Cl2��+H2��+2OH-
C.������Mg(HCO3)2��Һ�ӹ�����Ba(OH)2��Һ�����ӷ���ʽΪ�� Mg2++2![]() +2Ba2++4OH-��2BaCO3��+Mg(OH)2��+2H2O
+2Ba2++4OH-��2BaCO3��+Mg(OH)2��+2H2O
D.HS����ˮ�ⷽ��ʽ��HS-+H2OH3O++S2-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ʯ����Ҫ�ɷ���BaCO3(��Ca2+��Mg2+��Fe3+������)��ʵ�������øÿ�ʯ�Ʊ�BaCl2��2H2O��������ͼ��
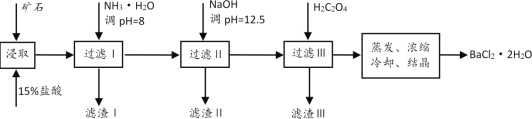
(1)��ϡ�����ȡǰ������ĥ��ʯ��Ŀ����__��
(2)����NH3��H2O����pH=8�ɳ�ȥ__(�����ӷ���)���������к�__(�ѧʽ)��
Ca2+ | Mg2+ | Fe3+ | |
��ʼ����ʱ��pH | 11.9 | 9.1 | 1.9 |
��ȫ����ʱ��pH | 13.9 | 11.1 | 3.2 |
(3)ҪʹCa2+��ȫ������Ӧ������Һ�е�![]() ��Ũ�Ȳ�����__mol/L(����Ũ��С��1��10-5mol/Lʱ����Ϊ�����ʹ���ȫ)��ͬʱ����H2C2O4ʱ��Ӧ���������ԭ����__��(��֪��KSP(BaC2O4=1.6��10-7��KSP(CaC2O4=2.3��10-9)��
��Ũ�Ȳ�����__mol/L(����Ũ��С��1��10-5mol/Lʱ����Ϊ�����ʹ���ȫ)��ͬʱ����H2C2O4ʱ��Ӧ���������ԭ����__��(��֪��KSP(BaC2O4=1.6��10-7��KSP(CaC2O4=2.3��10-9)��
(4)����������ԭ�ζ����ɲⶨH2C2O4��Ũ�ȣ�ȡ20.00mLH2C2O4��Һ����ƿ�У���0.10mol/L����KMnO4��Һ�ζ���KMnO4��ҺӦװ��__(������ʽ�ζ�����������ʽ�ζ�����)�С�д����Ӧ�����ӷ���ʽΪ__���ζ��յ������Ϊ__�����ζ��ﵽ�յ�ʱ������KMnO4��Һ30.00mL����H2C2O4��Һ��Ũ��Ϊ__mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л���Ӧ����ʽ��д��ȷ��Ϊ�ӳɷ�Ӧ���ǣ� ��
A.CH4+Cl2![]() CH2Cl2+H2
CH2Cl2+H2
B.CH2=CH2+Br2��CH3-CHBr2
C.CH2=CH2��H2O![]() CH3CH2OH
CH3CH2OH
D.![]() +HNO3
+HNO3![]()
![]() +H2O
+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��������������ԭ�����͵���
A.��ˮ�д���ƽ�⣺Cl2+H2O![]() H++Cl��+HClO����������NaHCO3(s)����Һ��ɫ��dz
H++Cl��+HClO����������NaHCO3(s)����Һ��ɫ��dz
B.����ˮƿʱ�д������������
C.���ڷ�Ӧ��ϵCO(g)+NO2(g)![]() NO(g)+CO2(g)����ƽ����ϵ����ѹǿ��ʹ��ɫ����
NO(g)+CO2(g)����ƽ����ϵ����ѹǿ��ʹ��ɫ����
D.���ڷ�Ӧ2NO2(g)![]() N2O4(g)��H<0��ƽ����ϵ�����¶���ɫ����
N2O4(g)��H<0��ƽ����ϵ�����¶���ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����л����˵������ȷ����
A.���ࡢ��֬�������ʶ��ܷ���ˮ�ⷴӦ
B.����![]() ��Һ���Լ��𱽡��Ҵ�������
��Һ���Լ��𱽡��Ҵ�������
C.������ϩ������ʹ��ˮ��ɫ������ɫԭ����ͬ
D.����ʽΪ![]() ��ͬ���칹�干��4��(�����������칹)
��ͬ���칹�干��4��(�����������칹)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com