
������λͬѧ�ֱ��ò�ͬ�ķ�������100 mL 3.6 mol/L��ϡ���ᡣ
(1)������18 mol/L��Ũ����������Һ,��Ҫ�õ�Ũ��������Ϊ������������������
(2)��ѧ��:��ȡŨ����,С�ĵص���ʢ������ˮ���ձ���,�������,����ȴ�����º�ת�Ƶ�100 mL����ƿ��,��������ˮ���ձ�������ϴ��2��3��,ÿ��ϴ��ҺҲת�Ƶ�����ƿ��,Ȼ��С�ĵ�������ƿ����ˮ���̶��߶���,����ƿ��,�������µߵ�ҡ�ȡ�
�ٽ���Һת�Ƶ�����ƿ�е���ȷ������
������ʱ���ӿ̶���,��������ҺŨ����������(�ƫ����ƫС������Ӱ�족)��
��ϴ�Ӳ�����,��ϴ���ձ����ϴҺҲע������ƿ,��Ŀ������ ��
�۶��ݵ���ȷ�������� ��
���ý�ͷ�ι�������ƿ�м�ˮʱ,��С��Һ�泬���˿̶�,�����ķ�������������(�����)��
A.��������Һ��,ʹ��Һ����̶�������
BС�ļ�������ƿ,��������,ʹ��Һ����̶�������
C.���������һ������Ũ����
D.��������
(3)��ѧ��:��100 mL��Ͳ��ȡŨ����,��������С�ĵؼ�������ˮ,�������,����ȴ�����º�,�ټ���ˮ��100 mL�̶���,�ٽ�����ȡ�����Ϊ�˷��Ƿ���ȷ?������ȷ,ָ�����д���֮��:�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(8��)(1) 0��5 mol SO2��Լ���� ��ԭ�ӣ����� gSO3������ԭ������ȡ�
��2��������ͬ�� ��HCl����NH3����CO2����O2���������У����з�����Ŀ���ٵ���(�����) _ ��
��3����100mL 0��2 mol/L ��NaOH��Һ��������Һϡ�͵�200 mL������Һ��Na+�����ʵ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��120�桢101 kPa�����£���H2��CH4��CO��ɵĻ������a mL��ͨ��һ����(��Ϊx mL)����ʹ����ȫȼ�ա�
(1)��a mL���������ȫȼ��������ͬ���������������ҲΪa mL��(��x��a)����ԭ���������CH4����������� ��
(2)����ȫȼ�պ�����CO2��H2O(g)�����������ͬ������Ϊ2a mL����ԭ���������CH4����������� ����Ҫ�ⶨԭ���������H2�����������������֪����ͬ�����µ��������ݣ������� (��ѡ����ĸ)��
A��2a mL���������ܶ�
B������CO2����������
C������H2O(g)��������
(3)��ԭ���������ȫȼ��ʱ�����ɵ�������ֻ��CO2��H2O(g)����x��ȡֵ��Χ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1������������ˮ��Һ�ܵ��絫���ڷǵ���ʵ��� ������ţ����Ҵ� �ڰ��� ������ ���Ȼ��� ������ �ɱ� ��BaSO4 ����� ���������� ��CaO
��2��ijʵ����Ҫʹ��240ml 0.4mol/L CuSO4��Һ���õ������Ƹ�Ũ����Һ��Ҫʹ�õ�������������ƽ���ձ����������� �� ����Ҫ���� �˵����������Ƶ���ʧȥ���ֽᾧˮ�������Ƴ�����ҺŨ�� ������ƫ�ߡ�ƫ�ͻ���Ӱ�죩
��3���������й��Ŵ�����ٸ�Ŵ��ڻ��е�һ�����ϣ��仯ѧʽ��BaCuSi2O6���������������ʽ��ʾ����ɣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ϳɰ���ҵ���������õĦ�Fe��������Ҫ�ɷ���FeO��Fe2O3��
��1��ijFeO��Fe2O3������У������������ʵ���֮��Ϊ4��5������Fe2����Fe3�����ʵ���֮��Ϊ________��
��2����������Fe2����Fe3�������ʵ���֮��Ϊ1��2ʱ�����������ߣ���ʱ����������������������������Ϊ________����С����ʾ������2λС������
��3����Fe2O3Ϊԭ���Ʊ������������������м�������̼�ۣ��������·�Ӧ��2Fe2O3��C 4FeO��CO2����
4FeO��CO2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵ����Ϻ����ḻ��һ��Ԫ�أ�������(N2H4)�͵����ᶼ�ǵ�Ԫ�ص���Ҫ�⻯��ڹ�ũҵ�����������������ش����á�
(1)�ϳɰ����������Ĵ����������˹��̵���;�����Ի�ѧ��ҵ����Ҳ��������ҪӰ�졣
���ڹ̶�������ܱ������У��������»�ѧ��Ӧ��N2(g)��3H2(g)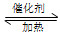 2NH3(g)����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3(g)����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
| T/K | 298 | 398 | 498 |
| ƽ�ⳣ��K | 4.1��106 | K1 | K2 |
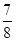 ����N2��ת����Ϊ________����NH3��Ũ�ȱ仯��ʾ�ù��̵ķ�Ӧ����Ϊ________��
����N2��ת����Ϊ________����NH3��Ũ�ȱ仯��ʾ�ù��̵ķ�Ӧ����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ������������⣺
��1��0.5 mol H2O������Ϊ g������____________�����ӡ�
��2�� 0.01molij���ʵ�����Ϊ1.08g��������ʵ�Ħ������Ϊ__________________��
��3������50 mL 0.2 mol��L��1 CuSO4��Һ����ҪCuSO4_____________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����m gij���壬������ԭ�ӷ��ӹ��ɣ�����Ħ������ΪM g��mol��1����
��1������������ʵ���Ϊ____ ____��
��2����������������ԭ������Ϊ________����
��3���������ڱ�״���µ����Ϊ________L��
��4������������1 Lˮ��(�����Ƿ�Ӧ)������Һ�����ʵ���������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������2.05 gij�ۺ�����ĸ��ε���ҺA�뺬1.20 g̼���ε���ҺB��ϣ�ǡ����ȫ��Ӧ������1.25 g��ɫ����C������ȥ����C����Һ�������õ���ɫ����D����������Dʱ��D�ֽ�ֻ��������̬���ʵĻ�����0 �桢1��105 Pa�£������Ϊ0.56 L�����õ�0.90 gҺ̬ˮ����һ����̬����Ϊ��̬������R2O���Իش�
(1)��ɫ����C�����ʵ���Ϊ________mol��
(2)A��Ħ������Ϊ__________��B��Ħ������Ϊ__________��
(3)R2O��H2O�����ʵ���֮��Ϊ__________������D������Ϊ________��D��Ħ������Ϊ________��R2O����Է�������Ϊ________��R2O�Ļ�ѧʽ��____________��
(4)д��A��B��ϵĻ�ѧ����ʽ_____________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com