
 ij�о���С�����A-D������װ������й�ʵ��
ij�о���С�����A-D������װ������й�ʵ��

 ��ʵ��һ���ռ�NO���塣
��ʵ��һ���ռ�NO���塣
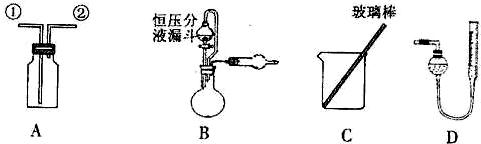
��1��  ��װ��A�ռ�NO���壬��ȷ�IJ����� ������ţ���
��װ��A�ռ�NO���壬��ȷ�IJ����� ������ţ��� a.�Ӣٿڽ���������ˮ������ b.�Ӣٿڽ�����������������
a.�Ӣٿڽ���������ˮ������ b.�Ӣٿڽ����������������� c.�Ӣڿڽ���������ˮ������ d..�Ӣڿڽ�����������������
c.�Ӣڿڽ���������ˮ������ d..�Ӣڿڽ����������������� ��ʵ�����Ϊ��̽����п�������ϵ�п����������
��ʵ�����Ϊ��̽����п�������ϵ�п���������� �ͶƲ��ȣ���ѯ��֪п�����ڼZn+2NaOH=Na2ZnO3+H2���ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g���ù����ռ��ˮ���Լ����������ʵ�鷽�����������ʵ�顣
�ͶƲ��ȣ���ѯ��֪п�����ڼZn+2NaOH=Na2ZnO3+H2���ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g���ù����ռ��ˮ���Լ����������ʵ�鷽�����������ʵ�顣 �����ף�ͨ������������Ӧ���ɵ����������ʵ��̽��ľĿ�ꡣ
�����ף�ͨ������������Ӧ���ɵ����������ʵ��̽��ľĿ�ꡣ ��2��ѡ��B�� ����������ţ�����װ�ý���ʵ�顣
��2��ѡ��B�� ����������ţ�����װ�ý���ʵ�顣 ��3����ó�ַ�Ӧ���������������ΪVL����״������
��3����ó�ַ�Ӧ���������������ΪVL����״������ = ��
= �� ��4������Ʋ��ȣ�����Ҫ������һ���������� ��
��4������Ʋ��ȣ�����Ҫ������һ���������� �� ��5����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©����������������ƫ����ƫС������Ӱ�족����
��5����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©����������������ƫ����ƫС������Ӱ�족���� �����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g ��
�����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g �� ��6��
��6�� ��
�� ��������ͨ������������Ӧǰ���������������Լ��������������ֵ��ΪH2��������ʵ��̽��Ŀ�ꡣʵ��ͬ��ʹ������C��
��������ͨ������������Ӧǰ���������������Լ��������������ֵ��ΪH2��������ʵ��̽��Ŀ�ꡣʵ��ͬ��ʹ������C�� ��7����ʵ�����Ƕȷ����������� �����ң�����ڡ��������ڡ���ͬ�ڡ�����
��7����ʵ�����Ƕȷ����������� �����ң�����ڡ��������ڡ���ͬ�ڡ�����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 65V |
| 22.4m1 |
| 2.9V |
| m1 |
| 65V |
| 22.4m1 |
| 2.9V |
| m1 |
| m1-m2 |
| m1 |
| m1-m2 |
| m1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(17��)
![]() ij�о���С�����A-D������װ������й�ʵ��
ij�о���С�����A-D������װ������й�ʵ��
![]()
![]() ��ʵ��һ���ռ�NO���塣
��ʵ��һ���ռ�NO���塣
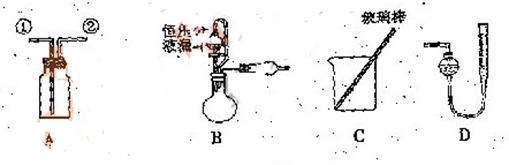
��1������ ![]() ��װ��A�ռ�NO���壬��ȷ�IJ����������� ������ţ���
��װ��A�ռ�NO���壬��ȷ�IJ����������� ������ţ���
![]() a.�Ӣٿڽ���������ˮ�������� ��������������b.�Ӣٿڽ�����������������
a.�Ӣٿڽ���������ˮ�������� ��������������b.�Ӣٿڽ�����������������
![]() c.�Ӣڿڽ���������ˮ���������������������� d..�Ӣڿڽ�����������������
c.�Ӣڿڽ���������ˮ���������������������� d..�Ӣڿڽ�����������������
![]() ��ʵ�����Ϊ��̽����п�������ϵ�п����������
��ʵ�����Ϊ��̽����п�������ϵ�п����������![]() �ͶƲ��ȣ���ѯ��֪п�����ڼZn+2NaOH=Na2ZnO3+H2���ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g���ù����ռ��ˮ���Լ����������ʵ�鷽�����������ʵ�顣
�ͶƲ��ȣ���ѯ��֪п�����ڼZn+2NaOH=Na2ZnO3+H2���ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g���ù����ռ��ˮ���Լ����������ʵ�鷽�����������ʵ�顣
![]() �����ף�ͨ������������Ӧ���ɵ����������ʵ��̽��ľĿ�ꡣ
�����ף�ͨ������������Ӧ���ɵ����������ʵ��̽��ľĿ�ꡣ
![]() ��2��ѡ��B���������� ����������ţ�����װ�ý���ʵ�顣
��2��ѡ��B���������� ����������ţ�����װ�ý���ʵ�顣
![]() ��3����ó�ַ�Ӧ���������������ΪVL����״������
��3����ó�ַ�Ӧ���������������ΪVL����״������![]() =������ ��
=������ ��
![]() ��4������Ʋ��ȣ�����Ҫ������һ������������������������ ��
��4������Ʋ��ȣ�����Ҫ������һ������������������������ ��
![]() ��5����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©����������������ƫ����ƫС������Ӱ�족����
��5����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©����������������ƫ����ƫС������Ӱ�족����
![]() �����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g ��
�����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g ��
![]() ��6��
��6��![]() ���������� ��
���������� ��
![]() ��������ͨ������������Ӧǰ���������������Լ��������������ֵ��ΪH2��������ʵ��̽��Ŀ�ꡣʵ��ͬ��ʹ������C��
��������ͨ������������Ӧǰ���������������Լ��������������ֵ��ΪH2��������ʵ��̽��Ŀ�ꡣʵ��ͬ��ʹ������C��
![]() ��7����ʵ�����Ƕȷ������������������� �����ң�����ڡ��������ڡ���ͬ�ڡ�����
��7����ʵ�����Ƕȷ������������������� �����ң�����ڡ��������ڡ���ͬ�ڡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]() ij�о���С�����A-D������װ������й�ʵ��
ij�о���С�����A-D������װ������й�ʵ��
![]()

![]() ��ʵ��һ���ռ�NO���塣
��ʵ��һ���ռ�NO���塣
��1�� ![]() ��װ��A�ռ�NO���壬��ȷ�IJ����� ������ţ���
��װ��A�ռ�NO���壬��ȷ�IJ����� ������ţ���
![]() a.�Ӣٿڽ���������ˮ������ b.�Ӣٿڽ�����������������
a.�Ӣٿڽ���������ˮ������ b.�Ӣٿڽ�����������������
![]() c.�Ӣڿڽ���������ˮ������ d..�Ӣڿڽ�����������������
c.�Ӣڿڽ���������ˮ������ d..�Ӣڿڽ�����������������
![]() ��ʵ�����Ϊ��̽����п�������ϵ�п����������
��ʵ�����Ϊ��̽����п�������ϵ�п����������![]() �ͶƲ��ȣ���ѯ��֪п�����ڼZn+2NaOH=Na2ZnO3+H2���ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g���ù����ռ��ˮ���Լ����������ʵ�鷽�����������ʵ�顣
�ͶƲ��ȣ���ѯ��֪п�����ڼZn+2NaOH=Na2ZnO3+H2���ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g���ù����ռ��ˮ���Լ����������ʵ�鷽�����������ʵ�顣
![]() �����ף�ͨ������������Ӧ���ɵ����������ʵ��̽��ľĿ�ꡣ
�����ף�ͨ������������Ӧ���ɵ����������ʵ��̽��ľĿ�ꡣ
![]() ��2��ѡ��B�� ����������ţ�����װ�ý���ʵ�顣
��2��ѡ��B�� ����������ţ�����װ�ý���ʵ�顣
![]() ��3����ó�ַ�Ӧ���������������ΪVL����״������
��3����ó�ַ�Ӧ���������������ΪVL����״������![]() = ��
= ��
![]() ��4������Ʋ��ȣ�����Ҫ������һ���������� ��
��4������Ʋ��ȣ�����Ҫ������һ���������� ��
![]() ��5����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©����������������ƫ����ƫС������Ӱ�족����
��5����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©����������������ƫ����ƫС������Ӱ�족����
![]() �����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g ��
�����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g ��
![]() ��6��
��6��![]() ��
��
![]() ��������ͨ������������Ӧǰ���������������Լ��������������ֵ��ΪH2��������ʵ��̽��Ŀ�ꡣʵ��ͬ��ʹ������C��
��������ͨ������������Ӧǰ���������������Լ��������������ֵ��ΪH2��������ʵ��̽��Ŀ�ꡣʵ��ͬ��ʹ������C��
![]() ��7����ʵ�����Ƕȷ����������� �����ң�����ڡ��������ڡ���ͬ�ڡ�����
��7����ʵ�����Ƕȷ����������� �����ң�����ڡ��������ڡ���ͬ�ڡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��)ij�о���С�����A-D������װ�ã������ظ�ʹ�ã�����й�ʵ��
��ʵ��һ���ռ�NO���塣
��1����װ��A�ռ�NO���壬��ȷ�IJ����� ������ţ���
a.�Ӣٿڽ���������ˮ������ b.�Ӣٿڽ�����������������
c.�Ӣڿڽ���������ˮ������ d..�Ӣڿڽ�����������������
��ʵ�����������Ũ������KMnO4��Ӧ�õ���Cl2��
��2�������װ��A����������Cl2װ��ͼ��������ʢ��ҩƷ����ע�������������
��ʵ������Ϊ��̽����п�������ϵ�п����������w(Zn)�ͶƲ��ȣ���ѯ��֪п������ǿ�Zn��2NaOH=Na2ZnO2��H2�� �ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g����NaOH��Һ���Լ����������ʵ�鷽�����������ʵ�顣
�����ף�ͨ���������������Һ��Ӧ���ɵ����������ʵ��̽��Ŀ�ꡣ
��3��ѡ�� �� ����������ţ�����װ�ý���ʵ�顣
��4����ó�ַ�Ӧ���������������ΪVL����״������w(Zn)= ��
��5������Ʋ��ȣ�����Ҫ������һ���������� ��
��6����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©������������� ���ƫ����ƫС������Ӱ�족����
�����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g ��
��7��w(Zn)= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ������ѧ�ڵڶ����¿���ѧ���� ���ͣ�ʵ����
��11�֣� ij�о���С�����A-D������װ������й�ʵ��

��ʵ��һ���ռ�NO���塣
��1����װ��A�ռ�NO���壬��ȷ�IJ��� ������ţ���
a.�Ӣٿڽ���������ˮ������ b.�Ӣٿڽ�����������������
c.�Ӣڿڽ���������ˮ������ d..�Ӣڿڽ�����������������
��ʵ�����Ϊ��̽����п�������ϵ�п����������w(Zn)�ͶƲ��ȣ���ѯ��֪п�����ڼZn+2NaOH=Na2ZnO2+H2���ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g���ù����ռ��ˮ���Լ����������ʵ�鷽�����������ʵ�顣
�����ף�ͨ������������Ӧ���ɵ����������ʵ��̽��Ŀ�ꡣ
��2��ѡ��B�� ����������ţ�����װ�ý���ʵ�顣
��3����ó�ַ�Ӧ���������������ΪVL����״������w(Zn) = ��
��4����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©�������������_____���ƫ����ƫС������Ӱ�족����
�����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g ��
��5��w(Zn) ��
��������ͨ������������Ӧǰ���������������Լ��������������ֵ��ΪH2��������ʵ��̽��Ŀ�ꡣʵ��ͬ��ʹ������C��
��6����ʵ�����Ƕȷ����������� �����ң�����ڡ��������ڡ���ͬ�ڡ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com