
��֪�������£�A�����ҺpH=a��B�����ҺpH=b��
(1)��AΪ���ᣬBΪ������������a=3��b=11�����ߵ������ϣ���Һ��pHΪ ��
a.����7 b.����7 c.��7
(2)��AΪ���ᣬBΪ�������ƣ���a=4��b=12����ôA��Һ��ˮ�������������Ũ��Ϊ mol/L,B��Һ��ˮ�������������Ũ��Ϊ mol/L��
(3)��A�Ļ�ѧʽΪHR��B�Ļ�ѧʽΪMOH����a+b=14�����ߵ������Ϻ���Һ�Լ��ԡ�������Һ�бض���һ�������ܷ���ˮ�⣬��ˮ�ⷴӦ�����ӷ���ʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ��֤��һˮ�ϰ�(NH3��H2O)��������ʣ��ס��ҡ������˷ֱ�ѡ�������Լ�����ʵ�飺
0.010 mol��L��1��ˮ��0.1 mol��L��1 NH4Cl��Һ��NH4Cl���塢��̪��Һ��pH��ֽ������ˮ��
(1)����pH��ֽ���0.010 mol��L��1��ˮ��pHΪ10�����϶�һˮ�ϰ���������ʣ�����Ϊ��һ�����Ƿ���ȷ�� (���ȷ������ȷ��)����˵������ ��
(2)��ȡ��10 mL 0.010 mol��L��1��ˮ����pH��ֽ����pH��a��Ȼ��������ˮϡ����1 000 mL������pH��ֽ����pH��b����Ҫȷ��NH3��H2O��������ʣ���a��bӦ����ʲô��ϵ�� ��
(3)��ȡ��10 mL 0.010 mol��L��1��ˮ������2�η�̪��Һ���Էۺ�ɫ���ټ�������NH4Cl���壬��Һ��ɫ�� (����dz��)������Ϊ��һ�����ܷ�֤��NH3��H2O��������ʣ� (��ܡ���)����˵��ԭ�� ��
(4)����������ṩ���Լ��������һ�������ּ��ķ���֤��NH3��H2O��������ʣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ij�о���ѧϰС����ƵĶ�һ�ַϾɺϽ�ĸ��ɷ֣�����Cu��Fe��Si ���ֳɷ֣����з��롢���������õĹ�ҵ���̣�ͨ�������̽����ɷ�ת��Ϊ���õĵ��ʼ������
��֪��298Kʱ��Ksp[Cu(OH)2]=2.2��10-20��Ksp[Fe(OH)3]=4.0��10-38�� Ksp[Mn(OH)2] =1.9��10-13��
�����������̻ش��й����⣺
��1��������ָ���� ��
��2���������FeCl3��Һ�����п����漰�Ļ�ѧ����ʽ�� ��
��3�������Ļ�ԭ��Ӧ�� ��
��4��������Һb�м�������KMnO4��Һ������Ӧ�����ӷ���ʽΪ ��
������X mol/L KMnO4��Һ������Һb����ǡ�ý���Һ�е���������ȫ����ʱ����KMnO4��ҺYmL����������ú���ɫ����C������Ϊ g���ú�X��Y�Ĵ���ʽ��ʾ����
��5��������,����Һc�������Ľ���������Ũ����ȣ�����Һc����μ���KOH��Һ�������ֽ��������ӳ������Ⱥ�˳��Ϊ�� �� �� ��(�����������)
��6�����һ��������ö��Ե缫���һ��ʱ�����������B������ΪZ g��ͬʱ������������ռ��������������ȣ��������������ɵ����һ���������Ϊ L���ú�Z�Ĵ���ʽ��ʾ�����õ缫�ķ�ӦʽΪ .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ̼����(����ԼΪ98%)�к���Ca2����Mg2����Fe3����Cl����SO42�������ʣ��ᴿ�����������£�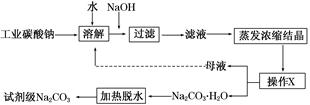
��.̼���Ƶı�����Һ�ڲ�ͬ�¶�����������������ͼ��ʾ��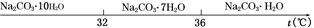
��.�й����ʵ��ܶȻ����£�
| ���� | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 | Fe(OH)3 |
| Ksp | 4.96��10��9 | 6.82��10��6 | 4.68��10��6 | 5.61��10��12 | 2.64��10��39 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ������ƿ�зֱ�װ�뺬��̪��0.01 mol��L-1 CH3COONa��Һ,���ֱ������ʢ��ˮ���ձ���,Ȼ�����ձ����м�����ʯ��,���ձ����м���NH4NO3����,�ձ����в����κ����ʡ�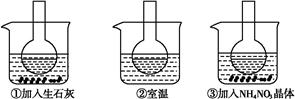
(1)����̪��0.01 mol��L-1 CH3COONa��Һ��dz��ɫ��ԭ��Ϊ
(2)ʵ������з�����ƿ������Һ��ɫ����,��ƿ������Һ��ɫ��dz,������������ȷ��������������
| A��ˮ�ⷴӦΪ���ȷ�Ӧ | B��ˮ�ⷴӦΪ���ȷ�Ӧ |
| C��NH4NO3����ˮʱ�ų����� | D��NH4NO3����ˮʱ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧѧϰС��Ϊ�о�HA��HB��MOH������Ե����ǿ��,�������ʵ��:�����½�pH=2����������ҺHA��HB��pH=12��MOH����Һ��1 mL,�ֱ��ˮϡ�͵�1 000 mL,��pH�ı仯����Һ����Ĺ�ϵ��ͼ,��������������,��ش���������:
(1)HAΪ ��,HBΪ ��(�ǿ��������)��
(2)��c=9,��ϡ�ͺ��������Һ��,��ˮ�����������Ũ�ȵĴ�С˳��Ϊ (���ᡢ�ѧʽ��ʾ)��
(3)��c=9,��ϡ�ͺ��HA��Һ��MOH��Һȡ��������,��������Һ��c(A-)��c(M+)�Ĵ�С��ϵΪc(A-) (����ڡ�����С�ڡ����ڡ�)c(M+)��
(4)��b+c=14,��MOHΪ ��(�ǿ��������)����ϡ�ͺ��HB��Һ��MOH��Һȡ��������,���û����Һ��pH 7(����ڡ�����С�ڡ����ڡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D�ֱ�ΪNH4HSO4��Ba(OH)2��AlCl3��Na2CO3 4�������е�1�֣�����ˮ����ȫ���룬�ֽ�������ʵ�飺
������A��Һ��B��Һ��Ϲ��ȿ����ɳ����ʹ̼�����ζ���壻
������A��Һ��C��Һ��Ͽ����ɳ����ң�
��A��Һ��B��Һ�����ܽ�����ң����������ܽ�����ס�
��ش�
(1)A�Ļ�ѧʽΪ________������ʱ����pH��ȵ�A��Һ��D��Һ�ֱ�ϡ��10����pH�ֱ��Ϊa��b����a________b(�>����������<��)��
(2)��������C��Һ�����գ�������ù���Ϊ________(�ѧʽ)��
(3)C��Һ��D��Һ��Ӧ�����ӷ���ʽΪ__________________________________________
(4)��B��Һ����μ��������������ʵ���Ũ�ȵ�NaOH��Һ���μӹ�����ˮ�ĵ���ƽ�⽫________(���������������)�ƶ�������������Һ�и�����Ũ���ɴ�С��˳��Ϊ________________________________________________________________________��
(5)��֪������Ksp��x����0.03 mol��L��1��A��Һ��0.01 mol��L��1��B��Һ�������ϣ������Һ��������ӵ�Ũ��Ϊ________(�ú�x�Ĵ���ʽ��ʾ����Ϻ���Һ����仯���Բ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Ԫ��(��ѧʽ��H2B��ʾ)��ˮ�еĵ��뷽��ʽ��H2B===H����HB����HB�� H����B2�����ش��������⡣
H����B2�����ش��������⡣
(1)Na2B��Һ��_____(����ԡ��������ԡ����ԡ�)��������_______(�����ӷ���ʽ��ʾ)��
(2)��0.1 mol��L��1��Na2B��Һ�У���������Ũ�ȹ�ϵʽ��ȷ����____��
A��c(B2��)��c(HB��)��c(H2B)��0.1 mol��L��1
B��c(Na��)��c(OH��)��c(H��)��c(HB��)
C��c(Na��)��c(H��)��c(OH��)��c(HB��)��2c(B2��)
D��c(Na��)��2c(B2��)��2c(HB��)
(3)��֪0.1 mol��L��1 NaHB��Һ��pH��2����0.1 mol��L��1 H2B��Һ�е������ӵ����ʵ���Ũ�ȿ���____0.11 mol��L��1(�<������>������)��������_____��
(4)0.1 mol��L��1 NaHB��Һ�и�������Ũ���ɴ�С��˳����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±��Ǽ���������ʵĵ���ƽ�ⳣ�������ܵ���ʵ��ܶȻ�Ksp��25�棩��
| ����� | ���뷽��ʽ | ���볣��K | Ksp |
| H2CO3 | H2CO3 HCO3����H�� HCO3����H��HCO3��  CO32����H�� CO32����H�� | K1��4.31��10��7 K2��5.61��10��11 | �� |
| C6H5OH | C6H5OH C6H5O����H�� C6H5O����H�� | 1.1��10��10 | �� |
| H3PO4 | H3PO4 H2PO4����H�� H2PO4����H��H2PO4��  HPO42����H�� HPO42����H��HPO42��  PO43����H�� PO43����H�� | K1��7.52��10��3 K2��6.23��10��6 K1��2.20��10��13 | �� |
| NH3��H2O | NH3��H2O OH����NH4�� OH����NH4�� | 1.76��10��5 | �� |
| BaSO4 | BaSO4��s�� Ba2����SO42�� Ba2����SO42�� | �� | 1.07��10��10 |
 C6H5OH��Һ��ˮ�ĵ���̶�
C6H5OH��Һ��ˮ�ĵ���̶�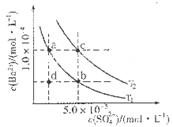
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com