
����ѧ����ѡ��2����ѧ�뼼������15�֣�
ͨ����ˮ�ܻ�õ�ˮ��ʳ�Ρ�þ�ȣ�ʳ�οɽ�һ�������ȼҵ����ش��������⡣
��1���о����ֺ�ˮ�����ķ�����_________��_________��
��2���ȼҵͨ����ⱥ��ʳ��ˮ�ܻ���ռ�����������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ_____________����ͼ�������ӽ���Ĥ����ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ������ʯī�ӵ�Դ_________�������ʱ���缫�ĵ缫��ӦʽΪ_________����������ͨ�����ӽ���Ĥ����Ҫ������__________��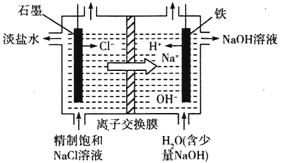
��3�������Ƽ�У�������ʳ��ˮ��ͨ��CO2��NH3�Ʊ�NaHCO3���仯ѧ����ʽΪ____________����ͨ�����__________���ѧʽ������������__________________ ��������NaHCO3���ȷֽ���Ʊ����
��4��Ŀǰ������60%���ϵ�þ���ǴӺ�ˮ����ȡ�ģ���֪��MgO��MgCl2���۵�ֱ�Ϊ2852���714�档����˵����ҵ�ϲ��õ������MgCl2�����ǵ������MgO������__________ ______________________________________________________________________ ��
��1���������ӽ�������������������ѡ��������2�֣�
��2��2NaCl+2H2O���2NaOH+H2��+Cl2����2�֣� ����1�֣� 2H++2e- H2����2�֣�
H2����2�֣�
Na+��H+��1�֣�
��3��NaCl+NH3+CO2+H2O NaHCO3+NH4Cl��2�֣� NH3��1�֣� NH3��������NaCl��Һ����CO2��NaCl��Һ���ܽ�Ⱥ�С��2�֣�
NaHCO3+NH4Cl��2�֣� NH3��1�֣� NH3��������NaCl��Һ����CO2��NaCl��Һ���ܽ�Ⱥ�С��2�֣�
��4�������۵�MgO��MgCl2�����MgO�ķѵ�࣬�����MgCl2�ĵ���٣�2�֣�
�������������
��1���������ӽ�������������������ѡ������
��2��2NaCl+2H2O���2NaOH+H2��+Cl2�� �� 2H++2e- H2��
H2��
Na+��H+
��3��NaCl+NH3+CO2+H2O NaHCO3+NH4Cl NH3 NH3��������NaCl��Һ����CO2��NaCl��Һ���ܽ�Ⱥ�С
NaHCO3+NH4Cl NH3 NH3��������NaCl��Һ����CO2��NaCl��Һ���ܽ�Ⱥ�С
��4�������۵�MgO��MgCl2�����MgO�ķѵ�࣬�����MgCl2�ĵ����
���㣺���黯ѧ�������е�Ӧ�á�


 �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д� �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����йؽ����Ĺ�ҵ�Ʒ��У���ȷ���ǣ� ��
| A����ͭ���û�ͭ����Ҫ�ɷ�ΪCuFeS2��ֱ�ӵ�⾫���õ�����Ϊ99.9%��ͭ |
| B����������ҵ�ϵ���Ȼ������Ʊ��� |
| C�����ƣ���ⱥ��NaCl��Һ |
| D����������CO�ڸ����»�ԭ����ʯ�е��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵���У�����ȷ����
| A���������ܴӺ�ˮ����ȡ��ˮ |
| B���Ӻ�ˮ�п��Եõ��Ȼ�þ���ټ��ȷֽ���ƽ���þ |
| C���������Ӻ�ˮ������Ĺؼ���Ӧ��Cl2+2Br��= 2Cl��+Br2 |
D��ú��������Ҫ��Ӧ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣�Ŀǰ������60%��þ�ǴӺ�ˮ��ȡ�ġ���ˮ��þ����Ҫ�������£�
��ʾ�� ��MgCl2����Ļ�ѧʽΪMgCl2?6H2O��
��MgO���۵�Ϊ2852�棬��ˮMgCl2���۵�Ϊ714�档
��1�������ٵ������� �� �����ڵ����� �� �����ˡ�
��2���Լ�a�������� ��
��3�����Ȼ�þ�����������þ�Ļ�ѧ����ʽΪ�� ��
��4����ҵ���ǽ�������þת��Ϊ�Ȼ�þ���ٵ���Ȼ�þ����ȡþ���ʣ��������ã�����Mg(OH)2�õ�MgO���ٵ������MgO�ķ����ƽ���þ����ԭ���� ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ���ˮ��þ����Ҫ��������:
��ش���������:
(1)�����ӷ�Ӧ�ĽǶ�˼��,�ں�ˮ�м���ʯ�������������������,д���ڳ������з�����Ӧ�����ӷ���ʽ����������������������������
(2)ʯ��������ʯ����ˮ�γɵĻ�����,�ӳ�����ú���ѧ��Դ,��߾���Ч��ĽǶ�,������ʯ�ҵ���Ҫԭ����Դ�ں����е�����������
(3)����A����������,����B������������
(4)����������Լ�a����������(�ѧʽ)��
(5)��ˮMgCl2������״̬��,ͨ�������Mg��Cl2,�÷�Ӧ�Ļ�ѧ����ʽΪ���������������ӿ��dzɱ��ͷ���ѭ�����õĽǶ�,����������������������������
(6)��ˮ��þ�Ĺ���,ΪʲôҪ����ˮ�е��Ȼ�þת��Ϊ������þ,��ת��Ϊ�Ȼ�þ?
_____________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ķ��������ݣ��ش����⡣
ұ������һ�����������ַ������ٽ�̿������ˮú��(��H2����CO)�����ۻ��ý����û������ܵ�ⷨ�����ַ���������ȱ�㣬�ڹ�ҵ�Ͼ���Ӧ�á�
(1)һ����ɫ����A����ȵ�̿��Ӧ���õ���һ����ɫ����B��B�����ȵ�����ͭ��Ӧ���ֵõ�A����A��B�ֱ�Ϊ_______(�����)��
A.O2��CO2 B.O2��CO C.CO2��CO D.CO��CO2
(2)����˵���������_______(�����)��
A.�ԷϾɽ�������ô��������ǻ���������
B.��������Ҫ������ʯ�ĸ�����ұ������������
C.���ý�����ұ������ͨ�����������Һ�Ƶ�
D.�Ȼ�ԭ���л�ԭ���н�̿��һ����̼����������ý�����
(3)��(Ti)�С�δ��������֮�ơ���ҵ�ϳ��ԣ�TiCl4+2Mg Ti+2MgCl2��ú���״�ѡ��÷�Ӧ���������ֻ����н���_______ (�����)��
Ti+2MgCl2��ú���״�ѡ��÷�Ӧ���������ֻ����н���_______ (�����)��
A.ϡ�������� B.������ C.������ D.CO2������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���Ի�����Ϊԭ������������Ҫ��Ϊ�����ν��У������ա������������ա���ش��������⣺
(1)���ջ������γɵ�¯�����뾭������ϴ�ӡ��������� (���豸����)������ҪĿ���� ��
(2)��������ʹ�õĴ�������ý(V2O5)�ܼӿ���������������ʣ��˹����в�����һ�������м���(��ͼ1)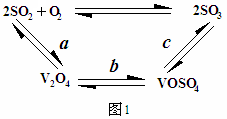
����a��c������Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ�� �� ��
(3)550��ʱ��SO2ת��ΪSO3��ƽ��ת����(��)����ϵ��ѹǿ(P)�Ĺ�ϵ��ͼ2��ʾ��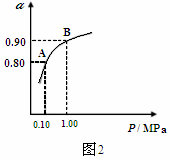
��2.0mol SO2��1.0mol O2����5L�ܱ������У���Ӧ��ƽ�����ϵ��ѹǿΪ0.10M Pa��A��B��ʾ��ͬѹǿ�µ�SO2ת���ʣ�ͨ������¹�ҵ�����в��ó�ѹ��ԭ���ǣ� ��
(4)Ϊѭ�����ô�����������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91.7%���ϡ���֪�Ϸ������к���V2O5��VOSO4�������Բ�������������֪��VOSO4������ˮ��V2O5������ˮ��NH4VO3������ˮ���ù��յ�������ͼ���£�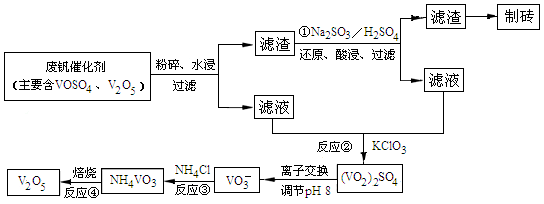
��Ӧ�٢ڢۢ�������������ԭ��Ӧ���� (���������)����Ӧ�ٵ����ӷ���ʽΪ ���ù����з�Ӧ�۵ij�����(�ֳƳ�����)�ǻ��շ��Ĺؼ�֮һ�������ʵĸߵͳ�����ҺpHӰ���⣬����Ҫ�����Ȼ��ϵ��(NH4Cl������������Һ��V2O5��������)���¶ȡ�������ͼ�Խ�������Ȼ��ϵ�����¶ȣ� �� ��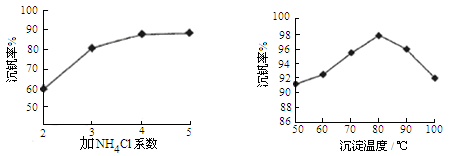
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�±��н�����ұ��ԭ���뷽������ȫ��ȷ���ǣ� ��
| | ұ��ԭ�� | ���� |
| A | 2HgO 2Hg��O2�� 2Hg��O2�� | �ȷֽⷨ |
| B | 2Al2O3�����ڣ� 4Al��3O2�� 4Al��3O2�� | ��ⷨ |
| C | Cu2S��O2 2Cu��SO2 2Cu��SO2 | �ȷֽⷨ |
| D | Fe2O3��2Al 2Fe��Al2O3 2Fe��Al2O3 | �Ȼ�ԭ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com