
�Ķ��������ݣ��ش����⡣
ұ������һ�����������ַ������ٽ�̿������ˮú��(��H2����CO)�����ۻ��ý����û������ܵ�ⷨ�����ַ���������ȱ�㣬�ڹ�ҵ�Ͼ���Ӧ�á�
(1)һ����ɫ����A����ȵ�̿��Ӧ���õ���һ����ɫ����B��B�����ȵ�����ͭ��Ӧ���ֵõ�A����A��B�ֱ�Ϊ_______(�����)��
A.O2��CO2 B.O2��CO C.CO2��CO D.CO��CO2
(2)����˵���������_______(�����)��
A.�ԷϾɽ�������ô��������ǻ���������
B.��������Ҫ������ʯ�ĸ�����ұ������������
C.���ý�����ұ������ͨ�����������Һ�Ƶ�
D.�Ȼ�ԭ���л�ԭ���н�̿��һ����̼����������ý�����
(3)��(Ti)�С�δ��������֮�ơ���ҵ�ϳ��ԣ�TiCl4+2Mg Ti+2MgCl2��ú���״�ѡ��÷�Ӧ���������ֻ����н���_______ (�����)��
Ti+2MgCl2��ú���״�ѡ��÷�Ӧ���������ֻ����н���_______ (�����)��
A.ϡ�������� B.������ C.������ D.CO2������
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���н���ұ���ķ�Ӧԭ����������ǣ� ��
A��2NaCl(����)  2Na+Cl2�� 2Na+Cl2�� | B��MgO+H2 Mg+H2O Mg+H2O |
C��Fe3O4+4CO  3Fe+4CO2 3Fe+4CO2 | D��2HgO 2Hg + O2�� 2Hg + O2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ����ѡ��2����ѧ�뼼������15�֣�
ͨ����ˮ�ܻ�õ�ˮ��ʳ�Ρ�þ�ȣ�ʳ�οɽ�һ�������ȼҵ����ش��������⡣
��1���о����ֺ�ˮ�����ķ�����_________��_________��
��2���ȼҵͨ����ⱥ��ʳ��ˮ�ܻ���ռ�����������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ_____________����ͼ�������ӽ���Ĥ����ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ������ʯī�ӵ�Դ_________�������ʱ���缫�ĵ缫��ӦʽΪ_________����������ͨ�����ӽ���Ĥ����Ҫ������__________��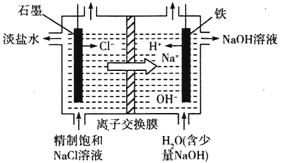
��3�������Ƽ�У�������ʳ��ˮ��ͨ��CO2��NH3�Ʊ�NaHCO3���仯ѧ����ʽΪ____________����ͨ�����__________���ѧʽ������������__________________ ��������NaHCO3���ȷֽ���Ʊ����
��4��Ŀǰ������60%���ϵ�þ���ǴӺ�ˮ����ȡ�ģ���֪��MgO��MgCl2���۵�ֱ�Ϊ2852���714�档����˵����ҵ�ϲ��õ������MgCl2�����ǵ������MgO������__________ ______________________________________________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
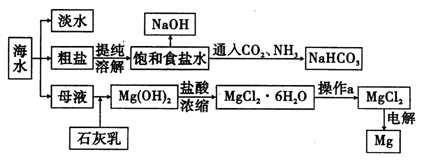
��ˮ��þ���ܴ���ԼΪ2.1��1015t��Ŀǰ������60%��þ���Ժ�ˮ����ҵ��ģ��ˮ��þ��������ͼ��ʾ��
�Իش��������⣺
(1)��ˮ��þ�Ĺ����Т١��ڷ�Ӧ�Ļ�ѧ����ʽ��
��_____________________________________��
��______________________________________��
(2)���Ȼ�þ��Һ��ȡ��ˮ�Ȼ�þ���壬�����������____________________
(3)Ϊ��ʹMgSO4��ȫת��ΪMg(OH)2����������ʯ��Ҫ������Ȼ������Mg(OH)2����������Ca(OH)2�ܽ�ȣ�Ӧ����________�����롣
(4)����þ�����Ĺ�ҵұ��������������֮�������в�֮ͬ���±�������þ���Ȼ�þ���۷е����ݣ�
| ���� | ����þ | �Ȼ�þ |
| �۵�(��) | 2 852 | 714 |
| �е�(��) | 3 600 | 1 412 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��(Ti)��Ϊ�������������Խ��Խ�������ǵĹ�ע�������£��Ѳ��ͷǽ�����ǿ�ᷴӦ������ʱ��ȴ���볣���ķǽ������ʷ�Ӧ�����Ǻ��ա������������ȷ���ı���ԭ�ϡ��ؿ��к��ѿ�ʯ֮һ�ƽ��ʯ(TiO2)Ŀǰ���ģ�����ѵķ����ǣ�
��һ�������ʯ��̼�ۻ�ϣ��ڸ���������ͨ�������Ƶ�TiCl4��һ�ֿ�ȼ�����塣�÷�Ӧ�Ļ�ѧ����ʽ��______________________���÷�Ӧ�Ļ�ԭ����________��
�ڶ�����������������У��ù�����þ�ڼ�����������TiCl4��Ӧ�Ƶý����ѡ�
(1)�˷�Ӧ�Ļ�ѧ����ʽ��__________________��
(2)�������������ò����л�ȡ�����ѵIJ���______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������˵������ȷ����____ ��
| A����������ijЩ���ַ����������� |
| B����������Ӳ�ȵ�ˮ��Ҫ�ü��ȵķ������������� |
| C�����Ṥҵ�У��ڽӴ��Ұ�װ�Ƚ�������Ϊ������S03ת��ΪH2S04ʱ�ų������� |
| D���ϳɰ���ҵԭ��������ʱ������̼�����Һ���ճ�ȥ������̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�����÷���м(����������������������)������ʽ������[Fe(OH)SO4]�Ĺ����������£�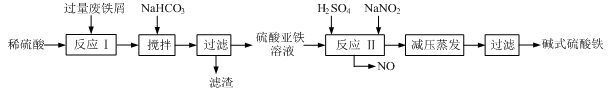
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�㶫ʡ���ŷḻ�ĺ�����Դ����ˮ��ȡʳ�κ�Br2�Ժ����±���������Ʊ�������MgCl2��MgO����±�к���Mg2����Cl��������������Na����Fe2����Fe3����CO(NH2)2�ȡ��Ʊ�������ͼ��ʾ��
(1)�����ijɷ���______(�ѧʽ)����Һ������������Ҫ�������� (д���ӷ���)��
(2)��NaClO��ȥ����CO(NH2)2ʱ������������⣬�����ܲ������ѭ�������ʣ���÷�Ӧ�Ļ�ѧ����ʽΪ________������NaClO������������______��
(3)ֱ�ӽ�MgCl2��6H2O��ǿ���ܵõ�MgO����Ӧ�Ļ�ѧ����ʽ��_____����MgCl2��6H2O�Ʊ���ˮMgCl2�����У�����Ҫ�Ļ�ѧ�Լ���________��
(4)��ˮ������������ճ�ʪ�����е�Br2������SO2���壬SO2����Br2�����ӷ���ʽ��_________________________________��SO2�������Դ�����Ṥҵ��β����ͬʱ��SO2β��Ҳ���ð�ˮ���գ���Ϊ�Ʊ����ʵ�ԭ�ϣ�SO2�����ð�ˮ���յõ��IJ��������________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com