

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?������һģ���������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
��2013?������һģ���������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
| ||
| ||
| ʵ���� | �� | �� | �� | �� |
| ��������g�� | 9.88 | 19.76 | 29.64 | 49.40 |
| Ũ�������ӵ�������g�� | m | m | 1.36 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | ��ʼʱ�����ʵ��� | ����ƽ��ʱ�ų���������QkJ�� | ƽ��ʱSO2ת���� ��X�� | |||
| SO2��mol�� | O2��mol�� | SO3��mol�� | N2 | |||
| �� | 2 | 1 | 0 | 0 | Q1 | X1 |
| �� | 1 | 0.5 | 0 | 0 | Q2=39.4 | X2 |
| �� | 1 | 0.5 | 0 | 1 | Q3 | X3 |
| �� | 1.8 | 0.9 | 0.2 | 0 | Q4 | X4 |

| t1-t2 | t3-t4 | t4-t5 | t6-t7 |
| K1 | K2 | K3 | K4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧʽ | HF | H2CO3 | HClO |
| ����ƽ�ⳣ�� ��Ka�� |
7.2��10-4 | K1=4.4��10-7 K2=4.7��10-11 |
3.0��10-8 |
| c(H+) |
| c(HF) |
| c(OH-) |
| c(H+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�걱����ʯ��ɽ��������һѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
����ƽ�ⳣ������Ka��ʾ)�Ĵ�С�����жϵ���ʵ����ǿ����25��ʱ���й����ʵĵ���ƽ�ⳣ�����±���ʾ��
|
��ѧʽ |
HF |
H2CO3 |
HClO |
|
����ƽ�ⳣ�� ��Ka�� |
7.2��10-4 |
K1=4.4��10-7 K2=4.7��10-11 |
3.0��10-8 |
��1����֪25��ʱ����HF(aq)+OH��(aq)��F��(aq)+H2O(l) ��H����67.7kJ/mol��
��H+(aq)+OH��(aq)��H2O(l) ��H����57.3kJ/mol ��
�����ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ________________________��
��2����Ũ��Ϊ0.1 mol/LHF��Һ��ˮϡ��һ���������¶Ȳ��䣩�����и����������____��
A��c(H+) B��c(H+)��c(OH��) C�� D��
D��
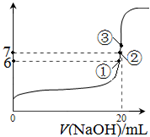
��3��25��ʱ����20mL0.1mol/L������м���VmL0.1mol/LNaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����_____��

A��pH��3��HF��Һ��pH��11��NaF��Һ�У� ��ˮ�������c(H+)���
B���ٵ�ʱpH��6����ʱ��Һ�У�c(F��)��c(Na+)��9.9��10-7mol/L
C���ڵ�ʱ����Һ�е�c(F��)��c(Na+)
D���۵�ʱV��20mL����ʱ��Һ��c(F��)< c(Na+)��0.1mol/L
��4�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L������������Һ�� �� Na2CO3��Һ �� NaHCO3��Һ �� NaF��Һ ��NaClO��Һ�����������ж�pH�ɴ�С��˳����______________��
��5��Na2CO3��Һ�Լ�������ΪCO32��ˮ���Ե�ʣ�����Ƽ�ʵ����ʵ֤��֮
___________________________________________________________��
��6������������һֱ��Ϊ���ĺ�������ڡ�1971��������ѧ���÷���ͨ��ϸ��ĩʱ���HFO����ṹʽΪH��O��F��HFO��ˮ��Ӧ�õ�HF�ͻ�����A��ÿ����1molHFת�� mol���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
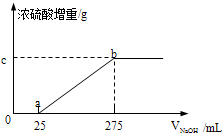 �������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
�������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����| ʵ���� | �� | �� | �� | �� |
| ��������g�� | 9.88 | 19.76 | 29.64 | 49.40 |
| Ũ�������ӵ�������g�� | m | m | 1.36 | 0 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com