
ijͬѧ����ʵ���о�ʱ��������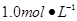 Ba(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�����ѧʽ����315������������������Һʱ������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���Ϊ̽��ԭ��ͬѧ���Ba(OH)2��8H2O��283K��293K��303Kʱ���ܽ�ȣ�g/100g H2O���ֱ�Ϊ2.5��3.9��5.6��
Ba(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�����ѧʽ����315������������������Һʱ������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���Ϊ̽��ԭ��ͬѧ���Ba(OH)2��8H2O��283K��293K��303Kʱ���ܽ�ȣ�g/100g H2O���ֱ�Ϊ2.5��3.9��5.6��
��1���ձ���δ�����ΪBaCO3��������
��2�������Լ��ɴ���Ba(OH)2��8H2O������BaCO3��ɣ����ʵ�鷽�������гɷּ��顣�ڴ����д��ʵ�鲽�衢Ԥ������ͽ��ۡ��������ǽᾧˮ�ļ��飻����ʱBaCO3������Һ��pH=9.6��
��ѡ�Լ���������ϡ���ᡢϡ���ᡢNaOH��Һ������ʯ��ˮ��pH�ơ��ձ����Թܡ����������ܡ��ι�
��3�����Լ������ᴿ��ȷ�ⶨ����Ba(OH)2��8H2O�ĺ�����ʵ�����£�
������250ml Լ Ba(OH)2��8H2O��Һ��ȷ��ȡw�������������ձ��У�����������ˮ�� ������Һת�� ��ϴ�ӣ����ݣ�ҡ�ȡ�
Ba(OH)2��8H2O��Һ��ȷ��ȡw�������������ձ��У�����������ˮ�� ������Һת�� ��ϴ�ӣ����ݣ�ҡ�ȡ�
�ڵζ���ȷ��ȡ25.00ml������Ba(OH)2��Һ����ƿ�У��μ�ָʾ������
���0.020������0.05������0.1980����1.5���� ����װ��50ml��ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ����������Vml��
����װ��50ml��ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ����������Vml��
�� ����Ba(OH)2��8H2O����������= ��ֻ�г���ʽ���������㣩
��4�������£�
(��ܡ����ܡ�) ����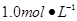 Ba(OH)2��Һ��
Ba(OH)2��Һ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ���Ϻ��г�����2013��߿�һģ��ѧ���� ���ͣ�058
ijͬѧ����ʵ���о�ʱ��������1.0 mol��L��1��Ba(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�(��ѧʽ����315)����������������Һʱ������ȡ�Լ���������ˮ�н������ܽ⣬�ձ��д��ڴ���δ���Ϊ̽��ԭ��ͬѧ���Ba(OH)2��8H2O��283 K��293 K��303 Kʱ���ܽ��(g/100 g��H2O)�ֱ�Ϊ2.5��3.9��5.6��
1���ձ���δ������ܽ�ΪBaCO3��������________��
2�������Լ��ɴ���Ba(OH)2��8H2O������BaCO3��ɣ����ʵ�鷽�������гɷּ��飮�ڴ���ֽ�Ͻ�һ�����ʵ�鲽�衢Ԥ������ͽ��ۣ�(�����ǽᾧˮ�ļ��飻����ʱBaCO3������Һ��pH��9.6)
��ѡ�Լ���������ϡ���ᡢϡ���ᡢNaOH��Һ������ʯ��ˮ��pH�ơ��ձ����Թܡ����������ܡ��ι�

���Լ������ᴿ��ȷ�ⶨ����Ba(OH)2��8H2O�ĺ�����ʵ�����¡�
3������250 mlԼ0.1 mol��L��1��Ba(OH)2��Һ��ȷ��ȡw�������������ձ��У�����������ˮ��________������Һת��________��ϴ�ӣ����ݣ�ҡ�ȣ�
4���ζ���ȷ��ȡ25.00 ml������Ba(OH)2��Һ����ƿ�У��μ�ָʾ������________(�0.020������0.05������0.1980����1.5��)mol��L��1����װ��50 ml��ʽ�ζ��ܣ��ζ����յ㣬��¼���ݣ��ظ��ζ�2�Σ�ƽ����������Vml������Ba(OH)2��8H2O������������________(ֻ�г���ʽ����������)
5�������£�________(��ܡ����ܡ�)����1.0 mol��L��1��Ba(OH)2��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ijͬѧ����ʵ���о�ʱ��������1.0mol•L-1Ba(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�����ѧʽ����315������������������Һʱ������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���Ϊ̽��ԭ��ͬѧ���Ba(OH)2��8H2O��283K��293K��303Kʱ���ܽ�ȣ�g/100g H2O���ֱ�Ϊ2.5��3.9��5.6��
��1���ձ���δ�����ΪBaCO3��������
��2�������Լ��ɴ���Ba(OH)2��8H2O������BaCO3��ɣ����ʵ�鷽�������гɷּ��顣�ڴ����д��ʵ�鲽�衢Ԥ������ͽ��ۡ����������ᾧˮ�ļ��飻����ʱBaCO3������Һ��pH=9.6��
��ѡ�Լ���������ϡ���ᡢϡ���ᡢNaOH��Һ������ʯ��ˮ��pH�ơ��ձ����Թܡ����������ܡ��ι�
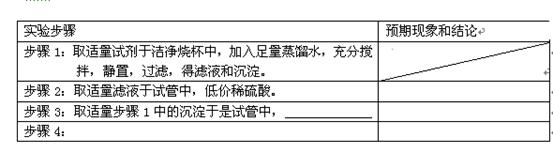
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ�����Լ��ڽྻ�ձ��У�������������ˮ����ֽ��裬���ã����ˣ�����Һ�ͳ����� |
|
| ����2��ȡ������Һ���Թ��У��ͼ�ϡ���ᡣ | |
| ����3��ȡ��������1�еij��������Թ��У� �� | |
| ����4�� |
��3�����Լ������ᴿ��ȷ�ⶨ����Ba(OH)2��8H2O�ĺ�����ʵ�����£�
������250ml Լ0.1mol•L-1Ba(OH)2��8H2O��Һ��ȷ��ȡw�������������ձ��У�����������ˮ�� ������Һת�� ��ϴ�ӣ����ݣ�ҡ�ȡ�
�ڵζ���ȷ��ȡ25.00ml������Ba(OH)2��Һ����ƿ�У��μ�ָʾ������ ���0.020������0.05������0.1980����1.5����mol•L-![]() 1����װ��50ml��ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ����������Vml��
1����װ��50ml��ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ����������Vml��
�� ����Ba(OH)2��8H2O����������= ��ֻ�г���ʽ���������㣩
��4�������£� (��ܡ����ܡ�) ����1.0 mol•L-1Ba(OH)2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ������ѧ����10�½��Բ��Ի�ѧ�Ծ� ���ͣ�ʵ����
��12�֣�ijͬѧ����ʵ���о�ʱ��������1��0 mol��L��1 Ba��OH��2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba��OH��2��8H2O�Լ�����ѧʽ����315������������������Һʱ������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���Ϊ̽����ԭ��ͬѧ���Ba��OH��2��8H2O��283 K��293 K��303 Kʱ���ܽ�ȣ�g/100 g H2O���ֱ�Ϊ2��5��3��9��5��6��
��1�������Լ��ɴ���Ba��OH��2��8H2O������BaCO3��ɡ����ʵ�鷽�������гɷּ��顣д��ʵ�鲽�衢Ԥ������ͽ��ۡ��������ǽᾧˮ�ļ��飻����ʱBaCO3������Һ��pH��9��6��
��ѡ�Լ���������ϡ���ᡢϡ���ᡢNaOH��Һ������ʯ��ˮ��pH�ơ��ձ����Թܡ����������ܡ��ι�
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ�����Լ��ڽྻ�ձ��У�������������ˮ����ֽ��裬���ã����ˣ�����Һ�ͳ����� | |
| ����2��ȡ������Һ���Թ��У��μ�ϡ���ᡣ | |
| ����3��ȡ��������1�еij������Թ��У�________�� | |
| ����4�� | |
 _������Һת��________�У�ϴ�ӣ����ݣ�ҡ�ȡ�
_������Һת��________�У�ϴ�ӣ����ݣ�ҡ�ȡ� ��ܡ����ܡ�������1��0 mol��L��1 Ba��OH��2��Һ��
��ܡ����ܡ�������1��0 mol��L��1 Ba��OH��2��Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ��г�����������ѧ����ĩ��ѧ�������л�ѧ�Ծ��������棩 ���ͣ�ʵ����
ijͬѧ����ʵ���о�ʱ������1.0mol•L-1Ba(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�����ѧʽ����315������������������Һʱ������ȡ�Լ���������ˮ�н������ܽ⣬�ձ��д��ڴ���δ���Ϊ̽��ԭ��ͬѧ���Ba(OH)2��8H2O��283K��293K��303Kʱ���ܽ�ȣ�g/100g H2O���ֱ�Ϊ2.5��3.9��5.6��
��1���ձ���δ������ܽ�ΪBaCO3��������___________________________________��
��2�������Լ��ɴ���Ba(OH)2��8H2O������BaCO3��ɣ����ʵ�鷽�������гɷּ��顣�ڴ���ֽ�Ͻ�һ�����ʵ�鲽�衢Ԥ������ͽ��ۡ��������ǽᾧˮ�ļ��飻����ʱBaCO3������Һ��pH=9.6��
��ѡ�Լ���������ϡ���ᡢϡ���ᡢNaOH��Һ������ʯ��ˮ��pH�ơ��ձ����Թܡ����������ܡ��ι�
|
ʵ�鲽�� |
Ԥ������ͽ��� |
|
|
|
|
����2��ȡ������Һ���Թ��У��μ�ϡ���ᡣ |
|
|
����3��ȡ��������1�еij������Թ��У�_____�� |
|
|
����4�� |
|
���Լ������ᴿ��ȷ�ⶨ����Ba(OH)2��8H2O�ĺ�����ʵ�����£�
��3������250ml Լ0.1mol•L-1Ba(OH)2��Һ��ȷ��ȡw�������������ձ��У�����������ˮ��__________������Һת��_____________��ϴ�ӣ����ݣ�ҡ�ȡ�
��4���ζ���ȷ��ȡ25.00ml������Ba(OH)2��Һ����ƿ�У��μ�ָʾ������__________���0.020������0.05������0.1980����1.5����mol•L-1����װ��50ml��ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ����������Vml�� ����Ba(OH)2��8H2O������������__________________��ֻ�г���ʽ���������㣩
��5�������£�________(��ܡ����ܡ�) ����1.0 mol•L-1Ba(OH)2��Һ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com