
�ڱ�״���£���aLNH3��ȫ����ˮ�õ�VmL��ˮ����Һ���ܶ�Ϊ��g��cm��3�����ʵ���������Ϊ�أ����ʵ����ʵ���Ũ��Ϊc mol/L��������������ȷ����
�� �أ�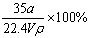 �� c��
�� c��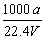
�� ������Һ���ټ���VmLˮ��������Һ��������������0��5��
�� ������Һ���ټ���1��5VmLͬŨ��ϡ���ᣬ��ַ�Ӧ����Һ������Ũ�ȴ�С��ϵΪ��
c��Cl������c��NH4+����c��H+����c��OH����
A���٢� B���ڢ� C���٢� D���ڢ�
 �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л������У�ͬ���칹����Ŀ����7������
A������ B����ϩ
C��1,2-������� D����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ʵ���֮��Ϊ1��3��п��ϡ������,�����ᱻ��ԭ�IJ���ΪN2O,��Ӧ������пû��ʣ��,����˵����ȷ����(�� ��)
A.�ڴ˷�Ӧ������ֻ����ǿ������
B.��Ӧ�����Һ���ټ��������,���ٷ�����ѧ��Ӧ
C.�÷�Ӧ�б���ԭ��������δ����ԭ������֮��Ϊ1��4
D.�÷�Ӧ�б���ԭ��������δ����ԭ������֮��Ϊ1��5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��д��ȷ����


A����������Һ�м������������������Һ��Al3+ + 2SO42��+ 2 Ba2+ + 4OH��= AlO2�� + 2BaSO4��+ 2H2O
B���������������м���������ϡ���Fe��OH��2+2H+ = Fe2++2H2O
C��������������Һ�м�������������������Һ��NH4++OH��=NH3?H2O
D����CH2BrCOOH�м�������������������Һ�����ȣ�
CH2BrCOOH+OH�� 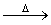 CH2BrCOO��+H2O
CH2BrCOO��+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ������ˮ��Һ�����ܴ����������������е������֣�K+��NH4+��Cl����Mg2+��Ba2+��CO32-��SO42-����ȡ����100mL��Һ��������ʵ�飺
��1����һ�ݼ���AgNO3��Һ�г�������
��2���ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04mol
��3�������ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g��
��������ʵ�飬�������ԭ��Һ���Ʋ���ȷ����
A��Cl��һ�������� B��K+һ������ C��Mg2+һ������ D��Ba2+���ܴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����KMnO4��H2O2��NaClO���������������ҽ���г�����������������H2O2��������Ư�ף��ǻ�ѧʵ������ر�����Ҫ�����Լ������������ɵ����տ��û�ԭ�ԵIJ��� (H2C2O4 )ȥ��Fe(NO3)3Ҳ����Ҫ�����Լ��������Ƕ����������������ʵ�̽����
(1)ijͬѧ�����ͭƬ��ϡ�����м���H2O2��ͭƬ�ܽ⣬д���÷�Ӧ�Ļ�ѧ����ʽ����˫���ŷ��ڻ�ѧ����ʽ�ϱ������ת�Ƶķ��������____________________��
(2)ȡ300 mL 0.2 mol/L��KI��Һ��һ����������KMnO4��Һǡ�÷�Ӧ�����ɵ����ʵ�����I2��KIO3��������KMnO4�����ʵ�������________mol��
(3)��Fe(NO3)3��Һ�м���Na2SO3��Һ����Һ�����ػ�ɫ��Ϊdz��ɫ����һ���ֱ�Ϊ�ػ�ɫ��д����Һ�ȱ�Ϊdz��ɫ�����ӷ���ʽ_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʽΪC5H7Cl���л����ṹ��������
A��ֻ����1��˫����ֱ���л��� B����2��˫����ֱ���л���
C����1��˫���Ļ�״�л��� D����һ��������ֱ���л���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й����ʷ���һ����ȷ���� ( )
��ǿ����ʣ��Ȼ��⡢����������Ħ���� ��������ʣ����ᡢ��������
�۷ǵ���ʣ�Һ������������ ��ͬϵ�CH2O2��C2H4O2��C3H6O2
A���٢ڢ� B���٢� C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����װ�á��Լ�ѡ�û������ȷ����
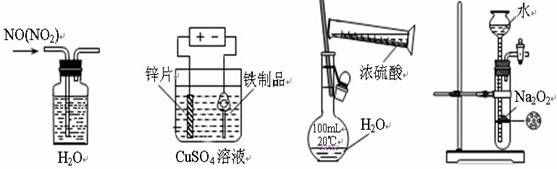
A����ȥNO�е�NO2 B������Ʒ�����п C��ϡ��Ũ���� D���Ʊ�����O2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com