
����Ŀ������ͼװ����ʾ��C��D��E��F��X��Y���Ƕ��Ե缫���ס�������Һ�������Ũ�ȶ���ͬ������ͨ��ǰ����Һ������䣩��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ��F�������ʺ�ɫ�� ��ش�

��1��B���ǵ�Դ��__________����C���ĵ缫��ӦʽΪ____________________________________��һ��ʱ�����X����������ɫ��______________�����������ߡ���dz����
��2�����ס���װ���е�C��D��E��F�缫��ֻ��һ�ֵ�������ʱ����Ӧ���ʵ����ʵ���֮��Ϊ ______________��
��3�����ñ�װ�ø�ͭ����������HӦ����______________ ������ͭ�����������������Һ��___________ ��Һ����������Һ��pH��13ʱ����ʱ����Һ���Ϊ500mL�������жƼ���������������Ϊ________ g��������Һ��pH _____________�����������������С����������������
��4�������ձ��������������ͭ����֪���ǰ���缫������ͬ�������ɺ�����ȡ����ϴ������ɡ����������ֶ����������5.12g������ʱ��·��ͨ���ĵ���Ϊ_______mol
���𰸡���4OH-�� 4 e����O2+2H2O��dz1��2��2��2ͭAgNO35.4��С0.08
��������
C��D��E��F��X��Y���Ƕ��Ե缫���ס�������Һ�������Ũ�ȶ���ͬ(����ͨ��ǰ����Һ�������)��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ����F�������ʺ�ɫ��˵��F�缫�����м���������F�缫�������ӷŵ�����������Ϊ����������C��E��G��X��������D��F��H��Y�����������������ĵ缫A�����������������ĵ缫B�Ǹ������ݴ˷�����
(1)������Ϸ��������Ե缫��ⱥ��NaCl��Һ���ܷ�Ӧ����ʽΪ��2NaCl+2H2O![]() 2NaOH+H2��+Cl2����F���Ժ�ɫ��˵��F�������������缫��Ӧ2H2O+2e-=2OH-+ H2������B�缫�ǵ�Դ�ĸ�����A�ǵ�Դ��������C���Դ������������C���������ö��Ե缫���CuSO4��Һʱ��ˮ�������OH-�������ŵ������������缫��ӦʽΪ4OH-�� 4 e����O2��+2H2O�����������������巢����Ӿ����Y�������������������������Ӵ�����ɣ���Y���ƶ�������X�缫��ɫ��dz��
2NaOH+H2��+Cl2����F���Ժ�ɫ��˵��F�������������缫��Ӧ2H2O+2e-=2OH-+ H2������B�缫�ǵ�Դ�ĸ�����A�ǵ�Դ��������C���Դ������������C���������ö��Ե缫���CuSO4��Һʱ��ˮ�������OH-�������ŵ������������缫��ӦʽΪ4OH-�� 4 e����O2��+2H2O�����������������巢����Ӿ����Y�������������������������Ӵ�����ɣ���Y���ƶ�������X�缫��ɫ��dz��
��ˣ�������ȷ����������4OH-�� 4 e����O2��+2H2O ����dz��
��2��C�缫��ӦΪ4OH-�� 4 e����O2��+2H2O��D�缫��ӦΪCu2++2e-=Cu��E�缫��ӦΪ2Cl��2e-=Cl2����F�缫��ӦΪ2H2O+2e-=2OH-+H2����һ��ʱ���ڣ��ĸ��缫ת�Ƶĵ�������ͬ�����Լס���װ�õ�C��D��E��F�缫���е������ɣ������ʵ���֮��Ϊ1:2:2:2��
��ˣ�������ȷ������1��2��2��2��
(3)��װ���ǵ�Ƴ����Ʋ�AgΪ�������Ƽ�CuΪ���������Һ����������Һ��G��������H������������G�ǶƲ�Ag��H�ǶƼ�Cu�����Һ����������Һ�������£�������Һ��pH=13����c(OH-)=0.1mol/L����Һ�����Ϊ500mL����n(OH-)=0.1mol/L��0.5L=0.05mol�����ݵ缫��Ӧʽ2H2O+2e-=2OH-+H2������֪ת�Ƶ��ӵ����ʵ���Ϊ0.05mol�����ݶ�����������Ӧʽ��Ag++e-=Ag����֪������������Ϊ0.05mol��108g/mol=5.4g����װ���Ƕ��Ե缫�������ͭ��Һ���ܷ�Ӧʽ2CuSO4+2H2O![]() 2Cu+2H2SO4+O2������Ӧ���������ᣬ���Լ�����Һ��pH��С��
2Cu+2H2SO4+O2������Ӧ���������ᣬ���Լ�����Һ��pH��С��
��ˣ�������ȷ������ͭ��AgNO3 ��5.4����С��
(4) ���ʱ�������Ͻ���ʧ���ӵ��½���������������������������������������������ƺ������������һ��Ϊ������������ͭ��������������������ͭ������=5.12g��![]() =2.56g���ɵ缫��ӦCu2++2e-=Cu֪��ת�Ƶ��ӵ����ʵ���=
=2.56g���ɵ缫��ӦCu2++2e-=Cu֪��ת�Ƶ��ӵ����ʵ���=![]() 0.08mol��
0.08mol��
��ˣ�������ȷ������0.08��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̽�������巴Ӧ�����屽��ʵ�������ͼ��ʾװ�ã�����װ�ò����������Ŀ��
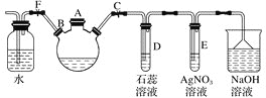
��1���ر�ֹˮ��F����ֹˮ��C����A����װ����������������ƿ�м������Һ�壬�ټ���������м����סA�ڣ���������ƿ�з�����Ӧ���л���ѧ����ʽΪ______________________��
��2��D��E�Թ��ڳ��ֵ�����ֱ�Ϊ��D.______�� E��__________��
��3����������ƿ�еķ�Ӧ���е���������ð��ʱ���ɿ�ֹˮ��F���ر�ֹˮ��C�����Կ�����������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����У�����ȥʳ��ˮ�е�ˮ����ѡ�� �� ��
A. �ձ� B. ������ C. ��Һ©�� D. ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ʣ���Ϊͬϵ�����
A. CH3CH=CH2��CH3CH3B.  ��
�� ![]()
C. CH3CH2OH ��CH2OHCH2OHD. C3H8��C5H12
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
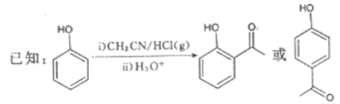
����Ŀ��G��ҩ��ϳ��е�һ����Ҫ�м��壬������G��һ�ֺϳ�·�ߣ�

�ش��������⣺
��1��B�Ľṹ��ʽΪ__________���������������ŵ�����Ϊ__________��B����C�ķ�Ӧ����Ϊ__________��
��2��D��������__________��
��3����C��E�ϳ�F�Ļ�ѧ����ʽΪ________________________________________��
��4��D��ͬ���칹���У��ܷ���������Ӧ�ҷ��ӽṹ�к������Ļ���__________�֣����к˴Ź�����������6��壬�����֮��Ϊ1��1��1��1��1��1��ͬ���칹��Ľṹ��ʽΪ______________________ (һ�ּ���)��
��5�����������ϳ�·�ߣ���CH3CH2ClΪԭ��(�����Լ���ѡ)������Ʊ��Ͷ�ȩ(CH3CH=CH
CHO)�ĺϳ�·�ߡ�_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��A����Է�������Ϊ84���ش��������⣺
(1)��A�ķ���ʽΪ_________������������A�����������ϣ��������ʵ���һ�������ȼ���������������������_____����������һ�������ȼ���������������������____��
A�� C7H8 B��C6H14 C��C7H14 D��C8H8
(2)����AΪ���������������е�̼ԭ����ͬһƽ���ϣ��÷��ӵ�һ��ȡ����ֻ��һ�֡���A�Ľṹ��ʽΪ__________����A����ʹ��ˮ��ɫ������һ�ȴ���ֻ��һ�֣���A�Ľṹ��ʽΪ__________ ������B����Է�����������AС6����BΪ������廯���д��B��Ũ���ᣬŨ�����Ϲ��ȵĻ�ѧ����ʽ__________
(3)��ͼ��

�ٸ�����������������ȫ�ӳɺ���һ�ȴ�����________�֣�
�ڸ����ʺ���ˮ��Ӧ������Br2�����ʵ���Ϊ_______mol��
�۸����ʺ�H2�ӳ���H2________mol��
(4)��ͼ�������һ�ֽṹM(ֻ������̼�ܣ�û�л�����ԭ��)������Ҫ�ش����⣺

����ϵͳ����������________��
��M��һ�ȴ�����________�֡�
��M����ijϩ���ӳ����ɵIJ�����ϩ��������_______�ֽṹ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��A�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ��
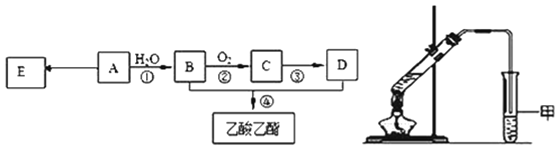
(1)0.1mol����A����______g�巢���ӳɷ�Ӧ���ӳɲ�����______mol��������ȫȡ����
(2)B�й����ŵ�������_________��Bͨ�����������ɵõ�D��Ҳ��ͨ������������Լ�Ϊ______����һ��ֱ������ΪD��
(3)E�dz����ĸ߷��Ӳ��ϣ�д��E�Ľṹ��ʽ__________���ϳ�E�ķ�Ӧ����_________��
(4)ijͬѧ����ͼ��ʾ��ʵ��װ����ȡ��������������ʵ��������Թܼ����ϲ�Ϊ���ġ�������ˮ����״Һ�塣
��ʵ�鿪ʼʱ���Թܼ��еĵ��ܲ�����Һ���µ�ԭ����__________________��
������ʵ���б���̼������Һ��������______________________��
���Ҵ������ᷴӦ�Ļ�ѧ����ʽ�ǣ�__________________�� Ũ�����������_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������������ʵ��Ӧ�ã���������������ԭ�����͵���
A. ��ˮ��������ƽ��Cl2+H2O ![]() HCl+ HClO��������AgNO3��Һ����Һ��ɫ��dz
HCl+ HClO��������AgNO3��Һ����Һ��ɫ��dz
B. �ϳɰ���ҵ��ʹ������ý������
C. �ϳ�NH3��Ӧ��Ϊ���NH3�IJ��ʣ�������Ӧ��ȡ���¶ȵĴ�ʩ
D. ��2HI��g��![]() H2(g)+I2(g)����������������䣬ͨ��������ʹ��ϵ��ɫ��dz
H2(g)+I2(g)����������������䣬ͨ��������ʹ��ϵ��ɫ��dz
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȡ![]() ij���ʷ���ʢ�������������ƹ���������У�Ȼ��ͨ������
ij���ʷ���ʢ�������������ƹ���������У�Ȼ��ͨ������![]() ���õ������ȼʹ���ַ�Ӧ�����Ƶù������������С��
���õ������ȼʹ���ַ�Ӧ�����Ƶù������������С��![]() �����������
�����������
A. ����![]() B. �ǻ�����
B. �ǻ�����![]()
C. �Ҵ�![]() D. ����
D. ����![]()
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com