
.A��B��C��DΪ������̬���ʡ���֪���� A��B�����ڷŵ������·������Ϸ�Ӧ������ﻹ������B�������ɺ���ɫ���壻
��C�ֱ���A��B��Ӧ���ɵ����ֻ��������E��F�ж�����10�����ӣ�
��C��D��Ӧ���ɵĻ�����J������ˮ��������Һ�еμ�AgNO3��Һ�����ɰ�ɫ������
��1��E��J �����ʵ���2��1ͨ��F�еõ�����Һ�д��ڵ��ĸ�ƽ����ϵ�����÷���ʽ��ʾ����
��֪����Һ�ʼ��ԣ���Һ������Ũ���ɴ�С��˳��Ϊ����������������������������
��2��A��C��D����Ԫ�ؿ����һ�����ӻ�����R���û�����R����ˮD��O�з���ˮ�ⷴӦ�Ļ�ѧ����ʽ�� ��
������H2�� H+����H-
NH����H��O
H+����H-
NH����H��O NH����H��O
NH����H��O
NH4+
+ H2O NH����H��O + H+��NH����H��O
NH����H��O + H+��NH����H��O NH4+ +��H-
NH4+ +��H-
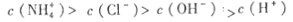
��2��NH4Cl+D2O NH����HDO + DCl
NH����HDO + DCl
�����������ݢٿ�֪��A�ǵ�����B�����������ݢڿ�֪��C��������E�ǰ�����F��ˮ�����ݢۿ�֪��D��������J���Ȼ��⡣
��1��E��J
�����ʵ���2��1��ϣ��������Ȼ�泥�ͬʱ����������������ˮ�е�ƽ����H2�� H+����H-��NH����H��O
H+����H-��NH����H��O NH����H��O��NH4+ + H2O
NH����H��O��NH4+ + H2O NH����H��O + H+��NH����H��O
NH����H��O + H+��NH����H��O NH4+ +��H-����Һ�ʼ��ԣ�˵����ˮ�ĵ���̶ȴ����Ȼ�淋�ˮ��̶ȣ���˹�ϵʽΪ
NH4+ +��H-����Һ�ʼ��ԣ�˵����ˮ�ĵ���̶ȴ����Ȼ�淋�ˮ��̶ȣ���˹�ϵʽΪ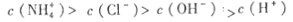 ��
��
��2��R���Ȼ�泥����ˮ����������������ӷ���ˮ�ⷴӦ����Ӧ����ʽΪNH4Cl+D2O NH����HDO + DCl��
NH����HDO + DCl��


 ����5��2���ϵ�д�
����5��2���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Al��OH��3
Al��OH��3 Al3++3OH-
Al3++3OH- Al��OH��3
Al��OH��3 Al3++3OH-
Al3++3OH-

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����a=3ʱ��e=2 | B����Ӧ�мȿ���ϡ�����ֿ���Ũ�����ữ | C��������11 g�������ʣ�����6.02��1023�����ӷ���ת�� | D����Ӧ������b��c��e�����¹�ϵ��3e=2b+c |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com