
(12��) ijʵ��С������ͼװ�ý����Ҵ���������ʵ�顣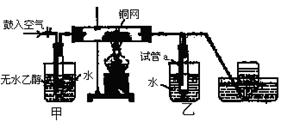
(1)ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ ��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ�� ��Ӧ�����Ȼ����ȣ���
(2)��������ˮԡ���ò���ͬ���������� ��
�ҵ������� ��
(3)��Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ������� ,
����ƿ���ռ������������Ҫ�ɷ��� ��
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л����� ��Ҫ��ȥ�����ʣ������ڻ��Һ�м��� (��д��ĸ)��Ȼ����ͨ�� (��ʵ���������)���ɳ�ȥ��
a���Ȼ�����Һ b���� c��̼��������Һ d�����Ȼ�̼

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
A��B��C��D�ֱ�������ֲ�ͬ��Ԫ�ء�Aԭ�ӵ����������Ų�Ϊns1��Bԭ�ӵļ۵����Ų�Ϊns2np2��Cԭ�ӵ�����������������Ӳ�����3����Dԭ�ӵ�L���Ӳ��p�����3�����ӡ�
��1��Cԭ�ӵĵ����Ų�ʽΪ ?? ����Aԭ�ӵ����������Ų�Ϊ1s1����ԭ�ӹ�����ص���ʽ�жϣ�A��C�γɵĻ������еĹ��ۼ��������� ?? ��A��C���γɵĻ�������۷е����Ը���A��C��ͬ����Ԫ�����γɵĻ�������۷е㣬��ԭ���� ?? ��
��2����n=2ʱ��Bԭ�ӵĽṹʾ��ͼΪ ?? ��B��C�γɵľ������� ���塣��n=3ʱ��B��C�γɵľ����У�Bԭ�ӵ��ӻ���ʽΪ ?? ���þ�������С�Ļ����� ?? �������ɣ�������������� ?? ��
��3����Aԭ�ӵ����������Ų�Ϊ4s1��Bԭ�ӵļ۵��Ų�Ϊ3s23p2��A��Ԫ�����ڱ��е�λ���� ?? ��A��B��C��D����Ԫ�صĵ�һ�������ɴ�С��˳����
?? ����Ԫ�ط��ű�ʾ����
B����ʵ�黯ѧ��12�֣�ij��ѧ�о���ѧϰС�������һϵ�С������ǵ�ľ̿��ȼ����ʵ�飬ʵ��װ������ͼ��ʾ��
 ��1������ͬѧ̽����ʹ������ľ̿��ȼʱO2����������ļ��ޡ����������Ϳ������ٶ�������O2���������Ϊ20%������ͬ������Ȼ�ϵ�100mL����A����ʵ�飬ʵ���¼���£�
��1������ͬѧ̽����ʹ������ľ̿��ȼʱO2����������ļ��ޡ����������Ϳ������ٶ�������O2���������Ϊ20%������ͬ������Ȼ�ϵ�100mL����A����ʵ�飬ʵ���¼���£�
| ��� | I | II | III | IV | V |
| V(O2)/mL | 60 | 40 | 20 | 12 | 10 |
| V(����)/mL | 40 | 60 | 80 | 88 | 90 |
| ���� | ľ̿��ȼ | ľ̿��ȼ | ľ̿��ȼ | ľ̿��ʱȼ�գ���ʱ��ȼ�� | ľ̿ ����ȼ |
�ش��������⣺
��ʹ������ľ̿��ȼ��O2�����������СԼΪ ?? ��
���ô�����ľ̿���鼯��ƿ���Ƿ���O2����ľ̿��ȼ���ܷ���Ϊ����ƿ�������һ���Ǵ�����O2���� ?? ����ܡ�����
��������3��2�ı������O2��CO2���壬�û�������ܷ�ʹ�����ǵ�ľ̿��ȼ��
�� ?? �����ܡ�������һ��������
��2������ͬѧ̽����NO2�ܷ�֧��ȼ�ա������⣬��������¼���ʵ�飬ʵ���¼���������£�
| ��� | ��ȡ����A�����з�Ӧ�����Ļ�����壩 | B�е����� | ���� |
| a | ����ƿ�м�������ŨHNO3 4HNO3 �� 4NO2��+O2��+2H2O�� | ��������ɫ���壬ľ̿����ȼ������Ϩ�� | NO2��֧��ȼ�� |
| b | ����AgNO3���� 2AgNO3 �� 2NO2��+O2��+2Ag | ��������ɫ���壬ľ̿��ȼ | NO2֧��ȼ�� |
| c | ����Cu(NO3)2���� 2Cu(NO3)2 �� 4NO2��+O2��+2CuO | ��������ɫ���壬ľ̿��ȼ | NO2֧��ȼ�� |
����Ϊa��b��c���������Ƿ�ɿ���˵��������ɡ�
a�� ?? ��ԭ���� ?? ��
b�� ?? ��ԭ���� ?? ��
c�� ?? ��ԭ���� ?? ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)ij��ѧ�о���ѧϰС��̽��Fe3+��SO32-֮�䷢�������ķ�Ӧ������һ����벢Э���������ʵ�顣
��������룺
��ͬѧ��Ϊ����������ԭ��Ӧ���䷴Ӧ�����ӷ���ʽΪ ��
��ͬѧ��Ϊ����˫ˮ�ⷴӦ���䷴Ӧ����ʽΪ2Fe3++3SO32-+6H2O=2Fe(OH)3(����)+3H2SO3��
��ʵ����֤��
��ͬѧ���������ʵ����̽����Ӧ�Ŀ����ԡ�
��Ϊ�˼�������Na2SO3�Ƿ���ʣ�Ӧѡ�õ��Լ��� ��
��ȡ5mLFeCl3��Һ���Թ��У���μ���Na2SO3��Һ���������۲쵽��Һ��ɫ�ɻ�ɫ��Ϊ����ɫ�������ݲ�����Ҳ�������ɣ���
�۽�����Һ�ֳ����ȷݣ�����һ�ݼ���ϡ�������������ټ���BaCl2ϡ��Һ���а�ɫ�������ɣ���һ�ݵ��뼸��KSCN��Һ����Һ���Ѫ��ɫ��
�ǵó����ۣ�
�ٸ��ݱ�ͬѧ��ʵ��ó��Ľ����ǣ� ��
��ʵ�������Һ���Ѫ��ɫ�����ӷ���ʽΪ ��
����չ̽����
�ٶ�ͬѧ��FeCl3��Һ�м���Na2CO3��Һ���۲쵽���ɫ�������Ҳ�����ɫ���壬�÷�Ӧ�����ӷ���ʽ�� ��
�ڴ���ʽ�Ͽ���Na2CO3��Na2SO3���ƣ����Ǵ�����ʵ���п��Կ��������ߵ�ˮ��Һ���Ȼ�����Һ��Ӧ��������ܴ�����ܵ�ԭ���SO32-ˮ��������CO32- �����С�����⣬����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��㶫ʡ½���и�һ��һ���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
(12��)ijͬѧ��ij�ִ��ν����ᴿʵ�飬�������ͼ����ش�
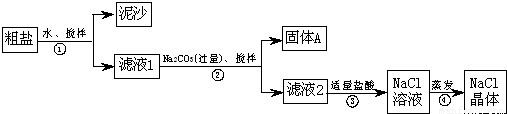
(1)����ٺ͢ڵIJ���������________��
(2)������жϼ������ᡰ�������ķ�����__________________________________������ܼ�������ʱҪ�ò��������Ͻ��裬����Ϊ�˷�ֹ________________�������������н϶����������ʱ��Ӧ________________��������ʹˮ�����ɣ�
(3)�������֤��
|
���� |
��֤�ķ��� |
���� |
���� |
|
�������A�к� CaCO3��MgCO3 |
ȡ��������A���Թ��У��μ�ϡ���ᣬ����Ϳ�г���ʯ��ˮ��С�ձ������Թܿ� |
____________ |
�������� |
|
�������A�к� BaCO3 |
ȡ��������A���Թ��У��ȵ���________���ٵ���Na2SO4��Һ |
�����ݷų����ް�ɫ���� |
___________
|
|
���������Ƶõ�NaCl�����л�����Na2SO4 |
ȡ����NaCl���������Թ��е�����ˮ��________ |
____________ |
�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ÿ��2�֣���12�֣�ij��ѧ�о���ѧϰС��̽��Fe3+��SO32�D֮�䷢�������ķ�Ӧ������һ����벢Э���������ʵ�顣
��������룺
��ͬѧ��Ϊ����������ԭ��Ӧ���䷴Ӧ�����ӷ���ʽΪ ��
��ͬѧ��Ϊ����˫ˮ�ⷴӦ���䷴Ӧ����ʽΪ2Fe3++3SO32�D+6H2O=2Fe(OH)3(����)+3H2SO3��
��ʵ����֤��
��ͬѧ���������ʵ����̽����Ӧ�Ŀ����ԡ�
��Ϊ�˼�������Na2SO3�Ƿ���ʣ�Ӧѡ�õ��Լ��� ��
��ȡ5mLFeCl3��Һ���Թ��У���μ���Na2SO3��Һ���������۲쵽��Һ��ɫ�ɻ�ɫ��Ϊ����ɫ�������ݲ�����Ҳ�������ɣ���
�۽�����Һ�ֳ����ȷݣ�����һ�ݼ���ϡ�������������ټ���BaCl2ϡ��Һ���а�ɫ�������ɣ���һ�ݵ��뼸��KSCN��Һ����Һ���Ѫ��ɫ��
�ǵó����ۣ�
�ٸ��ݱ�ͬѧ��ʵ��ó��Ľ����ǣ� ��
��ʵ�������Һ���Ѫ��ɫ�����ӷ���ʽΪ ��
����չ̽����
�ٶ�ͬѧ��FeCl3��Һ�м���Na2CO3��Һ���۲쵽���ɫ�������Ҳ�����ɫ���壬�÷�Ӧ�����ӷ���ʽ�� ��
�ڴ���ʽ�Ͽ���Na2CO3��Na2SO3���ƣ����Ǵ�����ʵ���п��Կ��������ߵ�ˮ��Һ���Ȼ�����Һ��Ӧ��������ܴ�����ܵ�ԭ���SO32��ˮ��������CO32��С�⣬���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����С�12��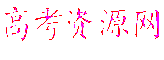 ��ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���
��ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���

�밴Ҫ����գ�
��1������Bװ�ÿ���ȡ��������______________________��д�����ּ��ɣ���
��2��A��C��E�������װ�ÿ�������ȡCl2��������ص�����ʵ�飮
���ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ��Ϊ���ݣ����Т�����ʵ
�飬ʵ����������������£�
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | ��Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��ˮ�м���̼�����Ʒ�ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ�IJ���������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com