
�ǻ������[Ca5(PO4)3OH]�����ݱ����һ���Ӳ���ʣ����ɱ������ݣ�������Һ�д�������ƽ�⣺Ca5(PO4)3OH(s)![]() 5Ca2+��3PO43����OH��
5Ca2+��3PO43����OH��
��ʳ��ϸ����ø������ʳ������л��ᣬ��ʱ���ݻ��ܵ���ʴ����ԭ����________����֪�������[Ca5(PO4)3F]���ܽ�ȱ�������ǻ�����Ƹ�С���������ӷ���ʽ��ʾ�������к��з�������ܷ�ֹȣ�ݵ�ԭ��________��
��������ԭ�����������һ�������ٽ����ݼ�̵ķ���______________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
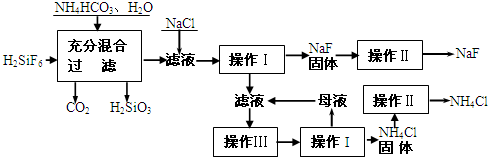
| �¶� | 10�� | 20�� | 30�� | �ܽ�ȣ�20��NaF-4g��0��NH4F-100g�� ����Na2SiF6-����ˮ |
| NH4Cl�ܽ�� | 33.3g | 37.2g | 41.4g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ�߶���ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ����
���ݱ��渲�ǵ������������������Ӳ�IJ��֣����ű������ݵ����ã�����Ҫ�ɷ�Ϊ�ǻ������[Ca5(PO4)3OH]�������ݱ������������ƽ�⣺�� ��
Ca5(PO4)3OH(s)  5Ca2+(aq)��3PO43-(aq)��OH- (aq) Ksp =
6.8��10-37 mol9��L-9
5Ca2+(aq)��3PO43-(aq)��OH- (aq) Ksp =
6.8��10-37 mol9��L-9
����˵��������ǣ� ��
A�������������ϵ��Ƿ��ͻ����H+���������������ȣ��
B��������ƽ���֪��С������ʱҪ�ٳ��Ƕಹ��
C������СOH-��Ũ�ȣ�����ƽ�⽫�����ƶ���Ksp��ֵ��Ӧ����
D��ʹ�ú��������ܷ�ֹȣ�ݣ�����ΪCa5(PO4)3OH(s)ת��Ϊ�����ܵ�Ca5(PO4)3F(s)[Ca5(PO4)3F(s)��Ksp = 2.8��10-61 mol9��L-9]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ�߶���һѧ���������⿼�Ի�ѧ�Ծ� ���ͣ�ѡ����
���ݱ��渲�ǵ������������������Ӳ�IJ��֣����ű������ݵ����ã�����Ҫ�ɷ�Ϊ�ǻ������[Ca5(PO4)3OH]�������ݱ������������ƽ�⣺
Ca5(PO4)3OH(s)  5Ca2+(aq)��3PO43-(aq)��OH- (aq) Ksp = 6.8��10-37 mol9��L-9
5Ca2+(aq)��3PO43-(aq)��OH- (aq) Ksp = 6.8��10-37 mol9��L-9
����˵���������
A�������������ϵ��Ƿ��ͻ����H+���������������ȣ��
B��������ƽ���֪��С������ʱҪ�ٳ��Ƕಹ��
C������СOH-��Ũ�ȣ�����ƽ�⽫�����ƶ���Ksp��ֵ��Ӧ����
D��ʹ�ú��������ܷ�ֹȣ�ݣ�����ΪCa5(PO4)3OH(s)ת��Ϊ�����ܵ�Ca5(PO4)3F(s)
[ Ca5(PO4)3F(s)��Ksp = 2.8��10-61 mol9��L-9]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ�����и߶���ѧ���������⿼�Ի�ѧ�Ծ� ���ͣ�ѡ����
���ݱ��渲�ǵ������������������Ӳ�IJ��֣����ű������ݵ����ã�����Ҫ�ɷ�Ϊ�ǻ������[Ca5(PO4)3OH]�������ݱ������������ƽ�⣺
Ca5(PO4)3OH(s)  5Ca2+(aq)��3PO43-(aq)��OH- (aq) Ksp =
6.8��10-37
mol9��L-9
5Ca2+(aq)��3PO43-(aq)��OH- (aq) Ksp =
6.8��10-37
mol9��L-9
����˵���������
A�������������ϵ��Ƿ��ͻ����H+���������������ȣ��
B��������ƽ���֪��С������ʱҪ�ٳ��Ƕಹ��
C������СOH-��Ũ�ȣ�����ƽ�⽫�����ƶ���Ksp��ֵ��Ӧ����
D��ʹ�ú��������ܷ�ֹȣ�ݣ�����ΪCa5(PO4)3OH(s)ת��Ϊ�����ܵ�Ca5(PO4)3F(s)
[ Ca5(PO4)3F(s)��Ksp = 2.8��10-61 mol9��L-9]
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com