
 �ƺ�ͭ�ĵ��ʼ��仯����������ʵ�������Ź㷺��Ӧ�ã�
�ƺ�ͭ�ĵ��ʼ��仯����������ʵ�������Ź㷺��Ӧ�ã�| NaF | NaCl | NaBr | NaI | |
| �۵�/�� | 993 | 801 | 747 | 661 |
���� ��1��CuԪ��ԭ�Ӻ��������Ϊ29�����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p43d104s1��Cuԭ��ʧȥ4s��3d�ܼ���1�������γ�Cu2+��
��2�����þ�̯�����㾧���к��е������Ӻ���������Ŀ��
��3�����Ӿ��������ӵİ뾶ԽС�����Ӽ�ǿ��Խ���۵�Խ�ߣ�
��4�����þ�̯�����㾧���к��е�ͭ���Ӻ͵�������Ŀ���õ�A�Ļ�ѧʽ���Ӷ�ȷ��ͭ�Ļ��ϼۣ�
��� �⣺��1��CuԪ��ԭ�Ӻ��������Ϊ29�����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1��Cuԭ��ʧȥ4s��3d�ܼ���1�������γ�Cu2+��Cu2+�ĺ�������Ų�ʽΪ��1s22s22p63s23p63d9��
�ʴ�Ϊ��1s22s22p63s23p63d9��
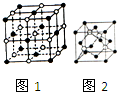
��2����ɫ���ʾ�����ӣ���ɫ���ʾ�����ӣ���������Ŀ=1+12��$\frac{1}{4}$=4����������Ŀ=$\frac{1}{8}$+6��$\frac{1}{2}$=4������ÿ��NaCl�����к��е�Na+���Ӻ�Cl-���ӵ���Ŀ�ֱ���4��4��һ����������Χ��6�������ӣ�������λ��Ϊ6���ʴ�Ϊ��4��6��
��3�����Ӿ��������ӵİ뾶ԽС�����Ӽ�ǿ��Խ���۵�Խ�ߣ������ӡ������ӡ������ӡ������ӵİ뾶��С������NaF��NaCL��NaBr��NaI����ľ����ܱ�С�������۵㽵�ͣ��ʴ�Ϊ�������ӡ������ӡ������ӡ������ӵİ뾶��С������NaF��NaCL��NaBr��NaI����ľ����ܱ�С�������۵㽵�ͣ�
��4��A�ľ�����ͼ2��ʾ����ɫ��ĸ�����8��$\frac{1}{8}$+6��$\frac{1}{2}$=4������Ϊ4��������A�Ļ�ѧʽ��CuI��A��ͭԪ����+1�ۣ��ʴ�Ϊ��CuI��+1��
���� ���⿼�������Ӻ�������Ų�������������Ŀ��������۵�ȣ�����Ӱ�쾧���۵�����ط�������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | AlCl3��Һ���ռ���Һ��Ӧ����n��OH-����n��Al3+��=7��2ʱ��2Al3++7OH-�TAl��OH��3��+AlO2-+2H2O | |
| B�� | ��ˮ����������SO2���壺NH3•H2O+SO2�TNH4++HSO3- | |
| C�� | ����SO2ͨ��NaClO��Һ�У�SO2+ClO-+H2O�TSO42-+Cl-+2H+ | |
| D�� | ��Fe��OH��3����Һ�м�������Fe��OH��3+3H+�TFe3++3H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��̼������Һ����ϴ������ | B�� | ��pH��ֽ���Բⶨ����������� | ||
| C�� | ���������Զ�����ˮɱ������ | D�� | �������������Ч��ֹȣ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ں�Al3+��H+����Һ�л����μ�NaOH��Һ��Al3+��H+ | |
| B�� | �ں�SiO32-����Һ�����μ�ϡ���SiO32-��CO32- | |
| C�� | �ں�Br-��Fe2+����Һ�л���ͨ��������Br-��Fe2+ | |
| D�� | �ں���Cu2+��Fe3+����Һ�м���п�ۣ�Cu2-��Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ϊs�������ʽ�����εģ����Դ���s����ϵĵ���������Բ���˶� | |
| B�� | ������3px��3py��3ps����������˶�ʱ��������ͬ | |
| C�� | ��������������������������˶�״̬�� | |
| D�� | H��F��Cl��O�ĵ縺�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
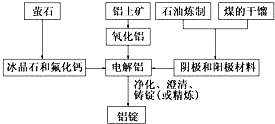 ��ͼ�Ǵ�ͳ�Ĺ�ҵ�����������Ļ�������ͼ�����������������ͼ�ش��������⣺
��ͼ�Ǵ�ͳ�Ĺ�ҵ�����������Ļ�������ͼ�����������������ͼ�ش��������⣺�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com