
| ʵ����� | 0.1000mol•L1 HCl��Һ���/mL | ����NaOH��Һ���/mL |
| 1 | 27.83 | 20.00 |
| 2 | 25.53 | 20.00 |
| 3 | 27.85 | 20.00 |
���� ���ȸ������ݵ���Ч�ԣ�Ȼ�����ƽ������V��NaOH�������Ÿ���c�����⣩=$\frac{c������V������}{V�����⣩}$�����㣻
��ʵ����������еĴ�����������Թ��Ϊ���ı���Һ����������������������ݸ���c�����⣩=$\frac{c������V������}{V�����⣩}$������ѡ�
��� �⣺�����εζ����ĵ����Ϊ��27.83mL��25.53mL��27.85mL����2���������ϴ���ȥ����ƽ������V��NaOH��=$\frac{27.83+27.85}{2}$ml=27.84mL��
c�����⣩=$\frac{c������V������}{V�����⣩}$=$\frac{0.1000mol/L��27.84��1{0}^{-3}L}{0.0200L}$=0.1392mol•L-1��
�ʴ�Ϊ��0.1392��
��A����ʽ�ζ���δ�ñ�������Һ��ϴ����Һ���ڱ�ˮĤϡ�ͣ����ı���Һ������ⶨŨ��ƫ�ߣ���A��ȷ��
B����ƿδ�ô���Һ��ϴ����ȷ�������ⶨ�����ȷ����B����
C������ζ��յ�ʱ�����Ӷ�������ȡ����Һ������ⶨ���ƫ�ߣ���C��ȷ��
D���ζ�ǰ���ζ����е���ҺҺ����͵��ڡ�0�������£�������ȷ�Բⶨ�����Ӱ�죬��D����
�ʴ�Ϊ��AC��
���� ������Ҫ�����˵ζ�����������������Ŀ�ѶȲ������ڿ���ѧ����ʵ��������������ݴ���������


 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
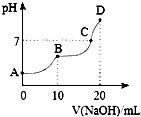 25��ʱ����10mL 0.1mol•L-1 H2C2O4��Һ�еμӵ�Ũ�ȵ�NaOH��Һ����Һ��pH��NaOH��Һ�������ϵ��ͼ��ʾ��������������ȷ���ǣ�������
25��ʱ����10mL 0.1mol•L-1 H2C2O4��Һ�еμӵ�Ũ�ȵ�NaOH��Һ����Һ��pH��NaOH��Һ�������ϵ��ͼ��ʾ��������������ȷ���ǣ�������| A�� | C����Һ�к���NaHC2O4��Na2C2O4 | |
| B�� | NaHC2O4��Һ��ˮ�ĵ���̶ȱ�Na2C2O4��Һ��С | |
| C�� | B�㣬c ��Na+��=2[c ��H2C2O4��+c ��HC2O4-��+c ��C2O42-��] | |
| D�� | D�㣬c ��Na+����c ��C2O42-����c ��OH-����c ��HC2O4-����c ��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���㽭ʡ��һ�ϵ�һ���ʼ컯ѧ���������棩 ���ͣ�ѡ����
���й������ӵļ��鼰����һ����ȷ����
A������ϡ�������������ɣ�������ͨ�����ʯ��ˮ����Һ����ǣ�����Һ��һ����CO32-
B������BaCl2��Һ�а�ɫ�������ɣ��ټ����ᣬ��������ʧ������Һ��һ����SO42-
C������Ũ����������Һ�����ȣ����ɵ�������ʹʪ��ĺ�ɫʯ����ֽ����������Һ��һ������NH4+
D������̼������Һ���ɰ�ɫ�������ټ������ɫ������ʧ������Һ��һ������Ba2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���㽭ʡ��һ�ϵ�һ���ʼ컯ѧ���������棩 ���ͣ�ѡ����
���е��뷽��ʽ�������
A��CaCl2 = Ca2++2Cl- B��Na2SO4=2Na+ +SO42-
C��HNO3=H++NO3- D��KClO3=K++C1-+3O2-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ�� �����Һ | A | B | C | D | E | F |
| 4mol•L-1H2SO4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O/mL | V7 | V8 | V9 | V10 | 10 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ����� | ������Һ���/ml | KMnO4��Һ���/ml | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 1.50 | 23.70 |
| 2 | 25.00 | 1.02 | 26.03 |
| 3 | 25.00 | 0.00 | 24.99 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
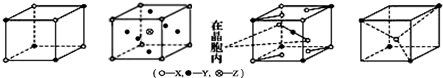
| A�� | XY | B�� | XY2Z | C�� | XY3 | D�� | XY2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ԣ���ԭ�ԣ������� | B�� | �������ԣ��л�ԭ�ԣ������� | ||
| C�� | �������ԣ��л�ԭ�ԣ������� | D�� | �������ԣ��л�ԭ�ԣ������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com