

 ���ɣ��ڸ���������ɵ������л��NH4HCO3���塢H2SiF6Ũ��Һ��H2Oʱ�������Լ���˳��Ӧ��
���ɣ��ڸ���������ɵ������л��NH4HCO3���塢H2SiF6Ũ��Һ��H2Oʱ�������Լ���˳��Ӧ��| 2000g |
| 100g |
| 2000g |
| 100g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
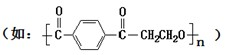
 ��2011?����ģ�⣩����3��1����������7��ί���Ϸ������棺�Խ���5��1���𣬽�ֹ��������������ӹ��������������������������������ͬʱָ������һ���㡱����Ҫ�ɷ�Ϊ�һ���ѿ�ӣ�������ζʳƷ�㾫��һ�֣��簴�ձ�ʹ�ö����������ģ����������������һ���ѿ�ӵĽṹ��ͼ���������й�˵����ȷ���ǣ�������
��2011?����ģ�⣩����3��1����������7��ί���Ϸ������棺�Խ���5��1���𣬽�ֹ��������������ӹ��������������������������������ͬʱָ������һ���㡱����Ҫ�ɷ�Ϊ�һ���ѿ�ӣ�������ζʳƷ�㾫��һ�֣��簴�ձ�ʹ�ö����������ģ����������������һ���ѿ�ӵĽṹ��ͼ���������й�˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com