
| 500�� |

| 500�� |
| 46��52.2% |
| 12 |
| 46��13.0% |
| 1 |
| 46��34.8% |
| 16 |
| Cu��Ag |
| �� |
| Cu��Ag |
| �� |


 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��1��������ֻ����̼��������Ԫ�أ��ڱ�״����Ϊ��̬���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ҵ�Ͽ����ñ����������������ʣ���Ӧʽ��ʾ��
��1��������ֻ����̼��������Ԫ�أ��ڱ�״����Ϊ��̬���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ҵ�Ͽ����ñ����������������ʣ���Ӧʽ��ʾ��| 500�� |
| Cu��Ag |
| �� |
| Cu��Ag |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
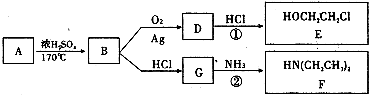
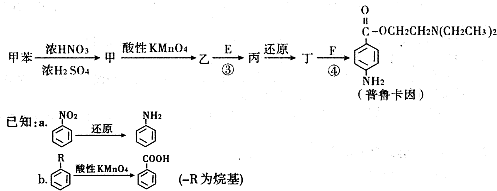
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��������ֻ����̼��������Ԫ�أ��ڱ�״����Ϊ��̬���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ҵ�Ͽ����ñ����������������ʣ���Ӧʽ��ʾ��
������������ C2H6 + �ף��������ʵĻ�ѧ��������Ϊ1:1:1��
��д�������ʿ��ܵ�ͬ���칹��Ľṹ��ʽ��������
������������������������������������������������������������������������
��2��ͨ����ʳ���Ϳɻ��ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%��
�� X�ķ���ʽΪ���������� ������ �������ŵ�����������������������
�� X������е�������ͭ���·�Ӧ����Y����ѧ����ʽΪ�������������������� ��
��3��X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ���������£�X��Z��Ӧ������һ������ζ������W��
 ijͬѧ������ͼ��ʾ��ʵ��װ����ȡW��ʵ��������Թܼ����ϲ�Ϊ���ġ�������ˮ����״Һ�塣
ijͬѧ������ͼ��ʾ��ʵ��װ����ȡW��ʵ��������Թܼ����ϲ�Ϊ���ġ�������ˮ����״Һ�塣
�� ʵ�鿪ʼʱ���Թܼ��еĵ��ܲ�����Һ���µ�ԭ���ǣ�
���������������������������������������������� ��
�� ��������Թܼ��и���״Һ����Ҫ�õ���������________������ţ���
a��©�������� b����Һ©���� c������©��
�� ʵ������������Թܼף�������ɫ�������ɣ�����Ҫԭ���ǣ�������������
���������������������������������������������������� ����ϻ�ѧ����ʽ�ش𣩡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�걱�����ص���ѧ��һ���£����л�ѧ�Ծ��������棩 ���ͣ������
 C2H6+�ף��������ʵĻ�ѧ��������Ϊ1��1��1��
C2H6+�ף��������ʵĻ�ѧ��������Ϊ1��1��1��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com